A Half-Century History of Applications of Antisense Oligonucleotides in Medicine, Agriculture and Forestry: We Should Continue the Journey
- PMID: 29844255
- PMCID: PMC6099785
- DOI: 10.3390/molecules23061302
A Half-Century History of Applications of Antisense Oligonucleotides in Medicine, Agriculture and Forestry: We Should Continue the Journey
Abstract
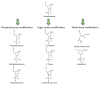
Antisense oligonucleotides (ASO), short single-stranded polymers based on DNA or RNA chemistries and synthesized in vitro, regulate gene expression by binding in a sequence-specific manner to an RNA target. The functional activity and selectivity in the action of ASOs largely depends on the combination of nitrogenous bases in a target sequence. This simple and natural property of nucleic acids provides an attractive route by which scientists can create different ASO-based techniques. Over the last 50 years, planned and realized applications in the field of antisense and nucleic acid nanotechnologies have produced astonishing results and posed new challenges for further developments, exemplifying the essence of the post-genomic era. Today the majority of ASOs are chemically modified and/or incorporated within nanoparticles to enhance their stability and cellular uptake. This review critically analyzes some successful cases using the antisense approach in medicine to address severe diseases, such as Duchenne muscular dystrophy and spinal muscular atrophy, and suggests some prospective directions for future research. We also examine in detail the elaboration of unmodified insect-specific DNA insecticides and RNA preparations in the areas of agriculture and forestry, a relatively new branch of ASO that allows circumvention of the use of non-selective chemical insecticides. When considering the variety of successful ASO modifications with an efficient signal-to-noise ratio of action, coupled with the affordability of in vitro oligonucleotide synthesis and post-synthesis procedures, we predict that the next half-century will produce a fruitful yield of tools created from effective ASO-based end products.
Keywords: DNA insecticides; RNAi; agriculture; antisense oligonucleotides; antisense therapy; forestry; medicine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Knorre D.G. Chemical instruments in modern biology (on the example of antisense effects on genetic structures) Soros Educ. J. 1998;12:25–31.
-
- Khorana H.G., Agarwal K.L., Buchi H., Caruthers M.H., Gupta N.K., Kleppe K., Kumar A., Otsuka E., RajBhandary U.L., Van de Sande J.H., et al. Studies on polynucleotides. 103. Total synthesis of the structural gene for an alanine transfer ribonucleic acid from yeast. J. Mol. Biol. 1972;72:209–217. doi: 10.1016/0022-2836(72)90146-5. - DOI - PubMed
-
- Caruthers M.H., Beaucage S.L., Efcavitch J.W., Fisher E.F., Matteucci M.D., Stabinsky Y. New chemical methods for synthesizing polynucleotides. Nucleic Acids Res. 1980;7:215–223. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical