Mechanisms responsible for increased circulating levels of galectin-3 in cardiomyopathy and heart failure
- PMID: 29844319
- PMCID: PMC5973942
- DOI: 10.1038/s41598-018-26115-y
Mechanisms responsible for increased circulating levels of galectin-3 in cardiomyopathy and heart failure
Abstract
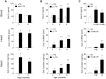
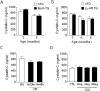
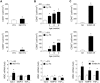
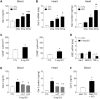
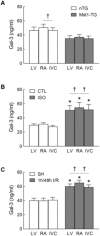
Galectin-3 is a biomarker of heart disease. However, it remains unknown whether increase in galectin-3 levels is dependent on aetiology or disease-associated conditions and whether diseased heart releases galectin-3 into the circulation. We explored these questions in mouse models of heart disease and in patients with cardiomyopathy. All mouse models (dilated cardiomyopathy, DCM; fibrotic cardiomyopathy, ischemia-reperfusion, I/R; treatment with β-adrenergic agonist isoproterenol) showed multi-fold increases in cardiac galectin-3 expression and preserved renal function. In mice with fibrotic cardiomyopathy, I/R or isoproterenol treatment, plasma galectin-3 levels and density of cardiac inflammatory cells were elevated. These models also exhibited parallel changes in cardiac and plasma galectin-3 levels and presence of trans-cardiac galectin-3 gradient, indicating cardiac release of galectin-3. DCM mice showed no change in circulating galectin-3 levels nor trans-cardiac galectin-3 gradient or myocardial inflammatory infiltration despite a 50-fold increase in cardiac galectin-3 content. In patients with hypertrophic cardiomyopathy or DCM, plasma galectin-3 increased only in those with renal dysfunction and a trans-cardiac galectin-3 gradient was not present. Collectively, this study documents the aetiology-dependency and diverse mechanisms of increment in circulating galectin-3 levels. Our findings highlight cardiac inflammation and enhanced β-adrenoceptor activation in mediating elevated galectin-3 levels via cardiac release in the mechanism.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

