In vivo wide-field calcium imaging of mouse thalamocortical synapses with an 8 K ultra-high-definition camera
- PMID: 29844612
- PMCID: PMC5974322
- DOI: 10.1038/s41598-018-26566-3
In vivo wide-field calcium imaging of mouse thalamocortical synapses with an 8 K ultra-high-definition camera
Abstract
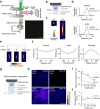
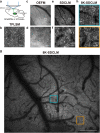
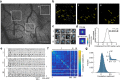
In vivo wide-field imaging of neural activity with a high spatio-temporal resolution is a challenge in modern neuroscience. Although two-photon imaging is very powerful, high-speed imaging of the activity of individual synapses is mostly limited to a field of approximately 200 µm on a side. Wide-field one-photon epifluorescence imaging can reveal neuronal activity over a field of ≥1 mm2 at a high speed, but is not able to resolve a single synapse. Here, to achieve a high spatio-temporal resolution, we combine an 8 K ultra-high-definition camera with spinning-disk one-photon confocal microscopy. This combination allowed us to image a 1 mm2 field with a pixel resolution of 0.21 µm at 60 fps. When we imaged motor cortical layer 1 in a behaving head-restrained mouse, calcium transients were detected in presynaptic boutons of thalamocortical axons sparsely labeled with GCaMP6s, although their density was lower than when two-photon imaging was used. The effects of out-of-focus fluorescence changes on calcium transients in individual boutons appeared minimal. Axonal boutons with highly correlated activity were detected over the 1 mm2 field, and were probably distributed on multiple axonal arbors originating from the same thalamic neuron. This new microscopy with an 8 K ultra-high-definition camera should serve to clarify the activity and plasticity of widely distributed cortical synapses.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
An Ultrastructural Study of the Thalamic Input to Layer 4 of Primary Motor and Primary Somatosensory Cortex in the Mouse.J Neurosci. 2017 Mar 1;37(9):2435-2448. doi: 10.1523/JNEUROSCI.2557-16.2017. Epub 2017 Jan 30. J Neurosci. 2017. PMID: 28137974 Free PMC article.
-
Hypocretin (orexin) induces calcium transients in single spines postsynaptic to identified thalamocortical boutons in prefrontal slice.Neuron. 2003 Sep 25;40(1):139-50. doi: 10.1016/s0896-6273(03)00598-1. Neuron. 2003. PMID: 14527439
-
Area-Specific Synapse Structure in Branched Posterior Nucleus Axons Reveals a New Level of Complexity in Thalamocortical Networks.J Neurosci. 2020 Mar 25;40(13):2663-2679. doi: 10.1523/JNEUROSCI.2886-19.2020. Epub 2020 Feb 13. J Neurosci. 2020. PMID: 32054677 Free PMC article.
-
Spike timing and synaptic dynamics at the awake thalamocortical synapse.Prog Brain Res. 2005;149:91-105. doi: 10.1016/S0079-6123(05)49008-1. Prog Brain Res. 2005. PMID: 16226579 Review.
-
Cerebello-thalamic synapses and motor adaptation.Cerebellum. 2002 Jan-Mar;1(1):69-77. doi: 10.1080/147342202753203104. Cerebellum. 2002. PMID: 12879975 Review.
Cited by
-
Large-scale cranial window for in vivo mouse brain imaging utilizing fluoropolymer nanosheet and light-curable resin.Commun Biol. 2024 Mar 4;7(1):232. doi: 10.1038/s42003-024-05865-8. Commun Biol. 2024. PMID: 38438546 Free PMC article.
-
A Novel Assay Allowing Drug Self-Administration, Extinction, and Reinstatement Testing in Head-Restrained Mice.Front Behav Neurosci. 2021 Oct 29;15:744715. doi: 10.3389/fnbeh.2021.744715. eCollection 2021. Front Behav Neurosci. 2021. PMID: 34776891 Free PMC article.
-
Whether or not to act is determined by distinct signals from motor thalamus and orbitofrontal cortex to secondary motor cortex.Nat Commun. 2025 Apr 4;16(1):3106. doi: 10.1038/s41467-025-58272-w. Nat Commun. 2025. PMID: 40185746 Free PMC article.
-
Pan-cortical 2-photon mesoscopic imaging and neurobehavioral alignment in awake, behaving mice.Elife. 2024 May 29;13:RP94167. doi: 10.7554/eLife.94167. Elife. 2024. PMID: 38808733 Free PMC article.
-
Disrupted calcium dynamics and electrophysiological activity in the stratum pyramidale and hippocampal alveus during fear conditioning in the 5xFAD model of Alzheimer's disease.Front Aging Neurosci. 2025 Aug 20;17:1550673. doi: 10.3389/fnagi.2025.1550673. eCollection 2025. Front Aging Neurosci. 2025. PMID: 40908955 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

