Emerging biomarkers in the diagnosis of prostate cancer
- PMID: 29844697
- PMCID: PMC5961643
- DOI: 10.2147/PGPM.S136026
Emerging biomarkers in the diagnosis of prostate cancer
Abstract
Prostate cancer (PCa) is the second most common cancer in men worldwide. A large proportion of PCa are latent, never destined to progress or affect the patients' life. It is of utmost importance to identify which PCa are destined to progress and which would benefit from an early radical treatment. Prostate-specific antigen (PSA) remains the most used test to detect PCa. Its limited specificity and an elevated rate of overdiagnosis are the main problems associated with PSA testing. New PCa biomarkers have been proposed to improve the accuracy of PSA in the management of early PCa. Commercially available biomarkers such as PCA3 score, Prostate Health Index (PHI), and the four-kallikrein panel are used with the purpose of reducing the number of unnecessary biopsies and providing information related to the aggressiveness of the tumor. The relationship with PCa aggressiveness seems to be confirmed by PHI and the four-kallikrein panel, but not by the PCA3 score. In this review, we also summarize new promising biomarkers, such as PSA glycoforms, TMPRSS2:ERG fusion gene, microRNAs, circulating tumor cells, androgen receptor variants, and PTEN gene. All these emerging biomarkers could change the management of early PCa, offering more accurate results than PSA. Nonetheless, large prospective studies comparing these new biomarkers among them are required to know their real value in PCa detection and prognosis.
Keywords: PCA3; PHI; PSA; four-kallikrein panel; miRNAs; prostate cancer.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures
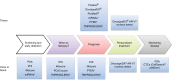
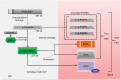
References
-
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. - PubMed
-
- Klotz L. Low-risk prostate cancer can and should often be managed with active surveillance and selective delayed intervention. Nat Clin Pract Urol. 2008;5(1):2–3. - PubMed
-
- D’Amico AV, Whittington R, Malkowicz SB, et al. Clinical utility of the percentage of positive prostate biopsies in defining biochemical outcome after radical prostatectomy for patients with clinically localized prostate cancer. J Clin Oncol. 2000;18(6):1164–1172. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

