Management of colonic diverticular disease in the third millennium: Highlights from a symposium held during the United European Gastroenterology Week 2017
- PMID: 29844795
- PMCID: PMC5964860
- DOI: 10.1177/1756284818771305
Management of colonic diverticular disease in the third millennium: Highlights from a symposium held during the United European Gastroenterology Week 2017
Abstract
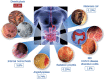
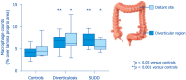
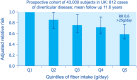
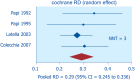
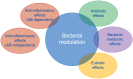
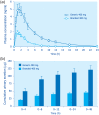
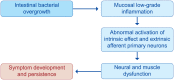
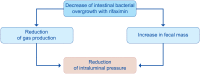
Diverticulosis is a common anatomical condition, which appears to be age-dependent. Individuals who develop chronic gastrointestinal symptoms or complications are referred to as having diverticular disease. Although the diagnosis of this condition can be relatively straightforward, randomized controlled trials are scarce and management often follows tradition rather than principles of evidence-based medicine. This report deals with the topics discussed during a symposium held during the United European Gastroenterology Week (Barcelona, October 2017). During the meeting, the role of dysbiosis in the pathogenesis of diverticular disease and its treatment were thoroughly discussed, by examining the efficacy and mechanisms of action of the currently used drugs. Recent studies have shown the presence of dysbiosis in patients with diverticular disease and suggest an imbalance in favor of bacteria with pro-inflammatory and pathogenetic potential. These microbiota changes correlate with mucosal immune activation, mirrored by a marked increase of macrophages in colonic mucosa, both in the diverticular region and at distant sites. The low-grade inflammation, driven by bacteria-induced immune activation, could be involved in the pathophysiology of symptoms. As a consequence, pharmacological approaches targeting enteric bacteria (with poorly absorbed antibiotics, like rifaximin, or probiotics) or intestinal inflammation (with 5-ASA derivatives or rifaximin) have shown capability of controlling symptoms and also preventing complications, albeit more research is needed to establish the optimal regimen (daily dose and duration) of therapy. Well-designed randomized-controlled trials (RCTs), including homogeneous populations of patients, are therefore needed. The future of management of many GI diseases, including symptomatic uncomplicated diverticular disease, will rely on the so-called 'microbiota-directed therapies'.
Keywords: diverticular disease; dysbiosis; mesalazine; microbiota; mucosal inflammation; probiotics; rifaximin.
Conflict of interest statement
Conflict of interest statement: Carmelo Scarpignato is member of the Speakers’ Bureau and of the Scientific Advisory Board of Alfasigma SpA. Giovanni Barbara is member of the Speakers’ Bureau of Alfasigma SpA. Angel Lanas is member of the Speakers’ Bureau and of the Scientific Advisory Board of Alfasigma SpA. Lisa L Strate has no conflicts of interest.
Figures






References
-
- Bevan R, Lee TJ, Nickerson C, et al. Non-neoplastic findings at colonoscopy after positive faecal occult blood testing: data from the English Bowel Cancer Screening Programme. J Med Screen 2014; 21: 89–94. - PubMed
-
- Strate LL, Modi R, Cohen E, et al. Diverticular disease as a chronic illness: evolving epidemiologic and clinical insights. Am J Gastroenterol 2012; 107: 1486–1493. - PubMed
-
- Cianci R, Iacopini F, Petruzziello L, et al. Involvement of central immunity in uncomplicated diverticular disease. Scand J Gastroenterol 2009; 44: 108–115. - PubMed
-
- Spiller RC, Sloan TJ. Do diverticula provide a unique niche for microbiota which can lead to activation of the innate immune system? Gut 2017; 66: 1175–1176. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

