Serotonin Selectively Increases Detectability of Motion Stimuli in the Electrosensory System
- PMID: 29845105
- PMCID: PMC5969320
- DOI: 10.1523/ENEURO.0013-18.2018
Serotonin Selectively Increases Detectability of Motion Stimuli in the Electrosensory System
Abstract
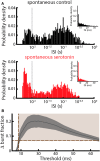
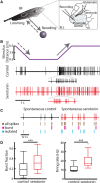
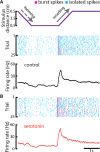
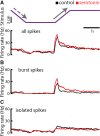
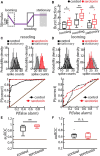
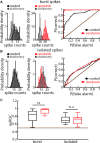
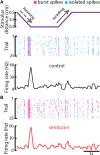
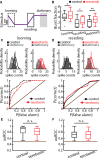
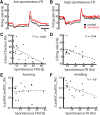
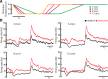
Serotonergic innervation of sensory areas is found ubiquitously across the central nervous system of vertebrates. Here, we used a system's level approach to investigate the role of serotonin on processing motion stimuli in the electrosensory system of the weakly electric fish Apteronotus albifrons. We found that exogenous serotonin application increased the firing activity of pyramidal neural responses to both looming and receding motion. Separating spikes belonging to bursts from those that were isolated revealed that this effect was primarily due to increased burst firing. Moreover, when investigating whether firing activity during stimulation could be discriminated from baseline (i.e., in the absence of stimulation), we found that serotonin increased stimulus discriminability only for some stimuli. This is because increased burst firing was most prominent for these. Further, the effects of serotonin were highly heterogeneous, with some neurons displaying large while others instead displaying minimal changes in responsiveness following serotonin application. Further analysis revealed that serotonin application had the greatest effect on neurons with low baseline firing rates and little to no effect on neurons with high baseline firing rates. Finally, the effects of serotonin on sensory neuron responses were largely independent of object velocity. Our results therefore reveal a novel function for the serotonergic system in selectively enhancing discriminability for motion stimuli.
Keywords: burst firing; feedback; neuromodulation; serotonin; weakly electric fish.
Figures
Similar articles
-
Serotonin modulates optimized coding of natural stimuli through increased neural and behavioural responses via enhanced burst firing.J Physiol. 2020 Apr;598(8):1573-1589. doi: 10.1113/JP278940. Epub 2020 Mar 3. J Physiol. 2020. PMID: 32011728
-
Serotonin selectively enhances perception and sensory neural responses to stimuli generated by same-sex conspecifics.Proc Natl Acad Sci U S A. 2013 Nov 26;110(48):19609-14. doi: 10.1073/pnas.1314008110. Epub 2013 Nov 11. Proc Natl Acad Sci U S A. 2013. PMID: 24218585 Free PMC article.
-
Plasticity in an electrosensory system. I. General features of a dynamic sensory filter.J Neurophysiol. 1996 Oct;76(4):2483-96. doi: 10.1152/jn.1996.76.4.2483. J Neurophysiol. 1996. PMID: 8899621
-
Neuromodulation of early electrosensory processing in gymnotiform weakly electric fish.J Exp Biol. 2013 Jul 1;216(Pt 13):2442-50. doi: 10.1242/jeb.082370. J Exp Biol. 2013. PMID: 23761469 Free PMC article. Review.
-
Ionic and neuromodulatory regulation of burst discharge controls frequency tuning.J Physiol Paris. 2008 Jul-Nov;102(4-6):195-208. doi: 10.1016/j.jphysparis.2008.10.019. Epub 2008 Oct 18. J Physiol Paris. 2008. PMID: 18992813 Free PMC article. Review.
Cited by
-
Serotonergic Modulation of Sensory Neuron Activity and Behavior in Apteronotus albifrons.Front Integr Neurosci. 2020 Jul 7;14:38. doi: 10.3389/fnint.2020.00038. eCollection 2020. Front Integr Neurosci. 2020. PMID: 32733214 Free PMC article.
-
Serotonin increases population coding of behaviorally relevant stimuli by enhancing responses of ON but not OFF-type sensory neurons.Heliyon. 2023 Jul 14;9(7):e18315. doi: 10.1016/j.heliyon.2023.e18315. eCollection 2023 Jul. Heliyon. 2023. PMID: 37539191 Free PMC article.
-
Novel Functions of Feedback in Electrosensory Processing.Front Integr Neurosci. 2019 Sep 13;13:52. doi: 10.3389/fnint.2019.00052. eCollection 2019. Front Integr Neurosci. 2019. PMID: 31572137 Free PMC article.
References
-
- Bastian J (1981) Electrolocation. II. The effects of moving objects and other electrical stimuli on the activities of two categories of posterior lateral line lobe cells in Apteronotus albifrons . J Comp Physiol A Neuroethol Sens Neural Behav Physiol 144:481–494.
-
- Bastian J (1982) Vision and electroreception. Integration of sensory information in the optic tectum of the weakly electric fish Apteronotus Albifrons. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 147:287–297. 10.1007/BF00609662 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
