Microwave Ablation in the Management of Colorectal Cancer Pulmonary Metastases
- PMID: 29845348
- PMCID: PMC6944322
- DOI: 10.1007/s00270-018-2000-6
Microwave Ablation in the Management of Colorectal Cancer Pulmonary Metastases
Abstract
Purpose: To review outcomes following microwave ablation (MWA) of colorectal cancer pulmonary metastases and assess predictors of oncologic outcomes.
Methods: Technical success, primary and secondary technique efficacy rates were evaluated for 50 patients with 90 colorectal cancer pulmonary metastases at immediate, 4-8 weeks post-MWA and subsequent follow-up CT and/or 18F-FDG PET/CT. Local tumor progression (LTP) rate, LTP-free survival (LTPFS), cancer-specific and overall survivals were assessed. Complications were recorded according to SIR classification.
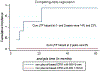
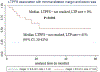
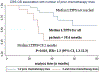
Results: Median follow-up was 25.6 months. Median tumor size was 1 cm (0.3-3.2 cm). Technical success, primary and secondary technique efficacy rates were 99, 90 and 92%, respectively. LTP rate was 10%. One-, 2- and 3-year LTPFS were: 93, 86 and 86%, respectively, with median LTPFS not reached. Median overall survival was 58.6 months, and median cancer-specific survival (CSS) was not reached. One-, 2- and 3-year overall and CSS were 94% and 98, 82 and 90%, 61 and 70%, respectively. On univariate analysis, minimal ablation margin (p < 0.001) and tumor size (p = 0.001) predicted LTPFS, with no LTP for minimal margin ≥ 5 mm and/or tumor size < 1 cm. Pleural-based metastases were associated with increased LTP risk (p = 0.002, SHR = 7.7). Pre-MWA CEA level > 10 ng/ml (p = 0.046) and ≥ 3 prior chemotherapy lines predicted decreased CSS (p = 0.02). There was no 90-day death. Major complications rate was 13%.
Conclusions: MWA with minimal ablation margin ≥ 5 mm is essential for local control of colorectal cancer pulmonary metastases. Pleural-based metastases and larger tumor size were associated with higher risk of LTP. CEA level and pre-MWA chemotherapy impacted CSS.
Keywords: Colorectal cancer; Lung ablation; Microwave ablation; Pulmonary metastases; Thermal ablation.
Conflict of interest statement
Figures

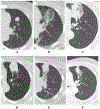
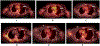



References
-
- Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–93. - PubMed
-
- Cook AD, Single R, McCahill LE. Surgical resection of primary tumors in patients who present with stage IV colorectal cancer: an analysis of surveillance, epidemiology, and end results data, 1988–2000. Ann Surg Oncol. 2005;12(8):637–45. - PubMed
-
- Kobayashi H, Mochizuki H, Sugihara K, Morita T, Kotake K, Teramoto T, et al. Characteristics of recurrence and surveillance tools after curative resection for colorectal cancer: a multicenter study. Surgery. 2007;141(1):67–75. - PubMed
-
- Mitry E, Guiu B, Cosconea S, Jooste V, Faivre J, Bouvier AM. Epidemiology, management and prognosis of colorectal cancer with lung metastases: a 30-year population-based study. Gut. 2010;59(10):1383–8. - PubMed
-
- Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104–17. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

