AKR1B10 activates diacylglycerol (DAG) second messenger in breast cancer cells
- PMID: 29846015
- PMCID: PMC6800193
- DOI: 10.1002/mc.22844
AKR1B10 activates diacylglycerol (DAG) second messenger in breast cancer cells
Abstract
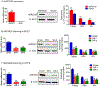
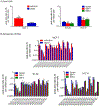
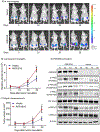
Aldo-keto reductase 1B10 (AKR1B10) is upregulated in breast cancer and promotes tumor growth and metastasis. However, little is known of the molecular mechanisms of action. Herein we report that AKR1B10 activates lipid second messengers to stimulate cell proliferation. Our data showed that ectopic expression of AKR1B10 in breast cancer cells MCF-7 promoted lipogenesis and enhanced levels of lipid second messengers, including phosphatidylinositol bisphosphate (PIP2), diacylglycerol (DAG), and inositol triphosphate (IP3). In contrast, silencing of AKR1B10 in breast cancer cells BT-20 and colon cancer cells HCT-8 led to decrease of these lipid messengers. Qualitative analyses by liquid chromatography-mass spectrum (LC-MS) revealed that AKR1B10 regulated the cellular levels of total DAG and majority of subspecies. This in turn modulated the phosphorylation of protein kinase C (PKC) isoforms PKCδ (Thr505), PKCµ (Ser744/748), and PKCα/βII (Thr638/641) and activity of the PKC-mediated c-Raf/MEK/ERK signaling cascade. A pan inhibitor of PKC (Go6983) blocked ERK1/2 activation by AKR1B10. In these cells, phospho-p90RSK, phospho-MSK, and Cyclin D1 expression was increased by AKR1B10, and pharmacological inhibition of the ERK signaling cascade with MEK1/2 inhibitors U0126 and PD98059 eradicated induction of phospho-p90RSK, phospho-MSK, and Cyclin D1. In breast cancer cells, AKR1B10 promoted the clonogenic growth and proliferation of breast cancer cells in two-dimension (2D) and three-dimension (3D) cultures and tumor growth in immunodeficient female nude mice through activation of the PKC/ERK pathway. These data suggest that AKR1B10 stimulates breast cancer cell growth and proliferation through activation of DAG-mediated PKC/ERK signaling pathway.
Keywords: AKR1B10; PKC/ERK cascade; breast cancer; diacylglycerol; lipid second messengers; phosphatidylinositol bisphosphate.
© 2018 Wiley Periodicals, Inc.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

