5-HT1F receptor regulates mitochondrial homeostasis and its loss potentiates acute kidney injury and impairs renal recovery
- PMID: 29846105
- PMCID: PMC6230742
- DOI: 10.1152/ajprenal.00077.2018
5-HT1F receptor regulates mitochondrial homeostasis and its loss potentiates acute kidney injury and impairs renal recovery
Abstract
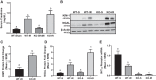
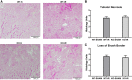
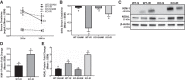
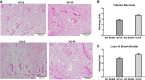
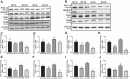
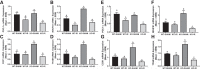
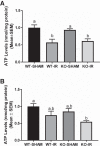
Our laboratory previously reported that agonists of the 5-hydoxytryptamine 1F (5-HT1F) receptor induce renal mitochondrial biogenesis (MB) and that stimulation of the 5-HT1F receptor following ischemia/reperfusion (I/R)-induced acute kidney injury (AKI) accelerated the recovery of renal function in mice. The goal of this study was to examine the contribution of the 5-HT1F receptor in the regulation of renal mitochondrial homeostasis and renal function in naïve and injured mice. Although 5-HT1F receptor knockout (KO) mice were healthy and fertile, and did not exhibit renal dysfunction, renal mitochondrial DNA copy number and mitochondrial fission gene expression increased at 10 wk of age. The 5-HT1F receptor KO mice exhibited greater proximal tubular injury and diminished renal recovery after I/R-induced AKI compared with wild-type mice. These findings were associated with persistent suppression of renal cortical MB and ATP levels after injury. In summary, the 5-HT1F receptor is a component of physiological MB regulation in the kidney, and its absence potentiates renal injury and impedes recovery.
Keywords: acute kidney injury; gene expression; mitochondria; serotonin.
Figures

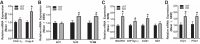
Similar articles
-
Agonism of the 5-hydroxytryptamine 1F receptor promotes mitochondrial biogenesis and recovery from acute kidney injury.J Pharmacol Exp Ther. 2014 Aug;350(2):257-64. doi: 10.1124/jpet.114.214700. Epub 2014 May 21. J Pharmacol Exp Ther. 2014. PMID: 24849926 Free PMC article.
-
Lasmiditan promotes recovery from acute kidney injury through induction of mitochondrial biogenesis.Am J Physiol Renal Physiol. 2023 Jan 1;324(1):F56-F63. doi: 10.1152/ajprenal.00249.2022. Epub 2022 Nov 3. Am J Physiol Renal Physiol. 2023. PMID: 36326468 Free PMC article.
-
The 5-hydroxytryptamine receptor 1F stimulates mitochondrial biogenesis and angiogenesis in endothelial cells.Biochem Pharmacol. 2019 Nov;169:113644. doi: 10.1016/j.bcp.2019.113644. Epub 2019 Sep 19. Biochem Pharmacol. 2019. PMID: 31542386 Free PMC article.
-
Serotonin regulation of mitochondria in kidney diseases.Pharmacol Res. 2024 May;203:107154. doi: 10.1016/j.phrs.2024.107154. Epub 2024 Mar 22. Pharmacol Res. 2024. PMID: 38521286 Free PMC article. Review.
-
Mitochondrial dysfunction and the AKI-to-CKD transition.Am J Physiol Renal Physiol. 2020 Dec 1;319(6):F1105-F1116. doi: 10.1152/ajprenal.00285.2020. Epub 2020 Oct 19. Am J Physiol Renal Physiol. 2020. PMID: 33073587 Review.
Cited by
-
Lasmiditan restores mitochondrial quality control mechanisms and accelerates renal recovery after ischemia-reperfusion injury.Biochem Pharmacol. 2023 Dec;218:115855. doi: 10.1016/j.bcp.2023.115855. Epub 2023 Oct 21. Biochem Pharmacol. 2023. PMID: 37866804 Free PMC article.
-
5-HT1F receptor agonism induces mitochondrial biogenesis and increases cellular function in brain microvascular endothelial cells.Front Cell Neurosci. 2024 Mar 5;18:1365158. doi: 10.3389/fncel.2024.1365158. eCollection 2024. Front Cell Neurosci. 2024. PMID: 38510106 Free PMC article.
-
Evidence-based review and frontiers of migraine therapy.Neurogastroenterol Motil. 2025 Mar;37(3):e14899. doi: 10.1111/nmo.14899. Epub 2024 Aug 12. Neurogastroenterol Motil. 2025. PMID: 39133210 Free PMC article. Review.
-
Serotonin system in the human placenta - the knowns and unknowns.Front Endocrinol (Lausanne). 2022 Dec 1;13:1061317. doi: 10.3389/fendo.2022.1061317. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36531448 Free PMC article. Review.
-
Renal Glomerular Mitochondria Function in Salt-Sensitive Hypertension.Front Physiol. 2020 Feb 4;10:1588. doi: 10.3389/fphys.2019.01588. eCollection 2019. Front Physiol. 2020. PMID: 32116733 Free PMC article.
References
-
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444: 337–342, 2006. doi:10.1038/nature05354. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

