A transcriptomics resource reveals a transcriptional transition during ordered sarcomere morphogenesis in flight muscle
- PMID: 29846170
- PMCID: PMC6005683
- DOI: 10.7554/eLife.34058
A transcriptomics resource reveals a transcriptional transition during ordered sarcomere morphogenesis in flight muscle
Abstract
Muscles organise pseudo-crystalline arrays of actin, myosin and titin filaments to build force-producing sarcomeres. To study sarcomerogenesis, we have generated a transcriptomics resource of developing Drosophila flight muscles and identified 40 distinct expression profile clusters. Strikingly, most sarcomeric components group in two clusters, which are strongly induced after all myofibrils have been assembled, indicating a transcriptional transition during myofibrillogenesis. Following myofibril assembly, many short sarcomeres are added to each myofibril. Subsequently, all sarcomeres mature, reaching 1.5 µm diameter and 3.2 µm length and acquiring stretch-sensitivity. The efficient induction of the transcriptional transition during myofibrillogenesis, including the transcriptional boost of sarcomeric components, requires in part the transcriptional regulator Spalt major. As a consequence of Spalt knock-down, sarcomere maturation is defective and fibers fail to gain stretch-sensitivity. Together, this defines an ordered sarcomere morphogenesis process under precise transcriptional control - a concept that may also apply to vertebrate muscle or heart development.
Keywords: D. melanogaster; Sarcomere; biomechanics; cell biology; development; developmental biology; muscle; self organization; stem cells; transcriptomics.
© 2018, Spletter et al.
Conflict of interest statement
MS, CB, AY, XZ, SL, AB, EB, GC, KB, BH, FS No competing interests declared
Figures
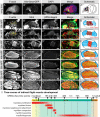
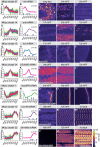

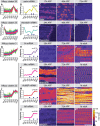
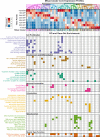







References
-
- Anant S, Roy S, VijayRaghavan K. Twist and notch negatively regulate adult muscle differentiation in Drosophila. Development. 1998;125:1361–1369. - PubMed
-
- Bate M, Rushton E, Currie DA. Cells with persistent twist expression are the embryonic precursors of adult muscles in Drosophila. Development. 1991;113:79–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

