Cell-Wall Recycling of the Gram-Negative Bacteria and the Nexus to Antibiotic Resistance
- PMID: 29847102
- PMCID: PMC6855303
- DOI: 10.1021/acs.chemrev.8b00277
Cell-Wall Recycling of the Gram-Negative Bacteria and the Nexus to Antibiotic Resistance
Abstract
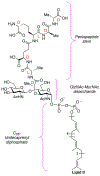
The importance of the cell wall to the viability of the bacterium is underscored by the breadth of antibiotic structures that act by blocking key enzymes that are tasked with cell-wall creation, preservation, and regulation. The interplay between cell-wall integrity, and the summoning forth of resistance mechanisms to deactivate cell-wall-targeting antibiotics, involves exquisite orchestration among cell-wall synthesis and remodeling and the detection of and response to the antibiotics through modulation of gene regulation by specific effectors. Given the profound importance of antibiotics to the practice of medicine, the assertion that understanding this interplay is among the most fundamentally important questions in bacterial physiology is credible. The enigmatic regulation of the expression of the AmpC β-lactamase, a clinically significant and highly regulated resistance response of certain Gram-negative bacteria to the β-lactam antibiotics, is the exemplar of this challenge. This review gives a current perspective to this compelling, and still not fully solved, 35-year enigma.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
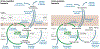
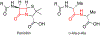

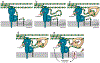



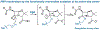
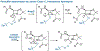





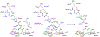

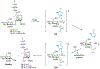
References
-
- van der Ploeg R; Verheul J; Vischer NOE; Alexeeva S; Hoogendoorn E; Postma M; Banzhaf M; Vollmer W; den Blaauwen T Colocalization and Interaction Between Elongasome and Divisome During a Preparative Cell Division Phase in Escherichia coli. Mol. Microbiol 2013, 87, 1074–1087. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

