Activationless Multiple-Site Concerted Proton-Electron Tunneling
- PMID: 29847111
- PMCID: PMC6310214
- DOI: 10.1021/jacs.8b04455
Activationless Multiple-Site Concerted Proton-Electron Tunneling
Abstract
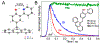
The transfer of protons and electrons is key to energy conversion and storage, from photosynthesis to fuel cells. Increased understanding and control of these processes are needed. A new anthracene-phenol-pyridine molecular triad was designed to undergo fast photoinduced multiple-site concerted proton-electron transfer (MS-CPET), with the phenol moiety transferring an electron to the photoexcited anthracene and a proton to the pyridine. Fluorescence quenching and transient absorption experiments in solutions and glasses show rapid MS-CPET (3.2 × 1010 s-1 at 298 K). From 5.5 to 90 K, the reaction rate and kinetic isotope effect (KIE) are independent of temperature, with zero Arrhenius activation energy. From 145 to 350 K, there are only slight changes with temperature. This MS-CPET reaction thus occurs by tunneling of both the proton and electron, in different directions. Since the reaction proceeds without significant thermal activation energy, the rate constant indicates the magnitude of the electron/proton double tunneling probability.
Conflict of interest statement
Notes
The authors declare no competing financial interest.
Figures

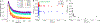
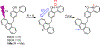
References
-
- Weinberg DR; Gagliardi CJ; Hull JF; Murphy CF; Kent CA; Westlake BC; Paul A; Ess DH; McCafferty DG; Meyer TJ Chem. Rev 2012, 112, 4016–4093. - PubMed
-
- Hammes-Schiffer S Chem. Rev 2010, 110, 6937–7100. - PubMed
-
- Biczok L; Linschitz HJ Phys. Chem 1995, 99, 1843–1845.
-
- Nomrowski J; Wenger OS Inorg. Chem 2015, 54, 3680–3687. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

