Biomimetic carbon monoxide delivery based on hemoglobin vesicles ameliorates acute pancreatitis in mice via the regulation of macrophage and neutrophil activity
- PMID: 29847178
- PMCID: PMC6058524
- DOI: 10.1080/10717544.2018.1477860
Biomimetic carbon monoxide delivery based on hemoglobin vesicles ameliorates acute pancreatitis in mice via the regulation of macrophage and neutrophil activity
Abstract
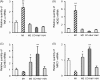
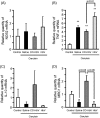
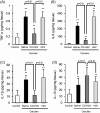
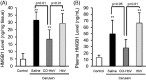
Macrophages play a central role in various inflammatory disorders and are broadly divided into two subpopulations, M1 and M2 macrophage. In the healing process in acute inflammatory disorders, shifting the production of M1 macrophages to M2 macrophages is desirable, because M1 macrophages secrete pro-inflammatory cytokines, whilst the M2 variety secrete anti-inflammatory cytokines. Previous findings indicate that when macrophages are treated with carbon monoxide (CO), the secretion of anti-inflammatory cytokine is increased and the expression of pro-inflammatory cytokines is inhibited, indicating that CO may have a potential to modulate the production of macrophages toward the M2-like phenotype. In this study, we examined the issue of whether CO targeting macrophages using a nanotechnology-based CO donor, namely CO-bound hemoglobin vesicles (CO-HbV), modulates their polarization and show therapeutic effects against inflammatory disorders. The results showed that the CO-HbV treatment polarized a macrophage cell line toward an M2-like phenotype. Furthermore, in an in vivo study using acute pancreatitis model mice as a model of an inflammatory disease, a CO-HbV treatment also tended to polarize macrophages toward an M2-like phenotype and inhibited neutrophil infiltration in the pancreas, resulting in a significant inflammation. In addition to the suppression of acute pancreatitis, CO-HbV diminished a subsequent pancreatitis-associated acute lung injury. This could be due to the inhibition of the systemic inflammation, neutrophil infiltration in the lungs and the production of HMGB-1. These findings suggest that CO-HbV exerts superior anti-inflammatory effects against inflammatory disorders via the regulation of macrophage and neutrophil activity.
Keywords: Macrophage; acute pancreatitis; carbon monoxide; liposome; polarization.
Figures



Similar articles
-
Carbon monoxide-bound hemoglobin vesicles ameliorate multiorgan injuries induced by severe acute pancreatitis in mice by their anti-inflammatory and antioxidant properties.Int J Nanomedicine. 2016 Oct 27;11:5611-5620. doi: 10.2147/IJN.S118185. eCollection 2016. Int J Nanomedicine. 2016. PMID: 27822039 Free PMC article.
-
Carbon monoxide alleviates endotoxin-induced acute lung injury via NADPH oxidase inhibition in macrophages and neutrophils.Biochem Pharmacol. 2025 Mar;233:116782. doi: 10.1016/j.bcp.2025.116782. Epub 2025 Jan 27. Biochem Pharmacol. 2025. PMID: 39880317
-
Carbon Monoxide Impairs CD11b+Ly-6Chi Monocyte Migration from the Blood to Inflamed Pancreas via Inhibition of the CCL2/CCR2 Axis.J Immunol. 2018 Mar 15;200(6):2104-2114. doi: 10.4049/jimmunol.1701169. Epub 2018 Feb 9. J Immunol. 2018. PMID: 29440506
-
Delivery strategies to control inflammatory response: Modulating M1-M2 polarization in tissue engineering applications.J Control Release. 2016 Oct 28;240:349-363. doi: 10.1016/j.jconrel.2016.01.026. Epub 2016 Jan 14. J Control Release. 2016. PMID: 26778695 Free PMC article. Review.
-
Macrophages in pancreatitis: Mechanisms and therapeutic potential.Biomed Pharmacother. 2020 Nov;131:110693. doi: 10.1016/j.biopha.2020.110693. Epub 2020 Sep 1. Biomed Pharmacother. 2020. PMID: 32882586 Review.
Cited by
-
Microenvironment of pancreatic inflammation: calling for nanotechnology for diagnosis and treatment.J Nanobiotechnology. 2023 Nov 23;21(1):443. doi: 10.1186/s12951-023-02200-x. J Nanobiotechnology. 2023. PMID: 37996911 Free PMC article. Review.
-
Carbon monoxide releasing molecule-A1 improves nonalcoholic steatohepatitis via Nrf2 activation mediated improvement in oxidative stress and mitochondrial function.Redox Biol. 2020 Jan;28:101314. doi: 10.1016/j.redox.2019.101314. Epub 2019 Aug 31. Redox Biol. 2020. PMID: 31514051 Free PMC article.
-
Drug discovery and formulation development for acute pancreatitis.Drug Deliv. 2020 Dec;27(1):1562-1580. doi: 10.1080/10717544.2020.1840665. Drug Deliv. 2020. PMID: 33118404 Free PMC article. Review.
-
Liposomal Artificial Red Blood Cell-Based Carbon Monoxide Donor Is a Potent Renoprotectant against Cisplatin-Induced Acute Kidney Injury.Pharmaceutics. 2021 Dec 27;14(1):57. doi: 10.3390/pharmaceutics14010057. Pharmaceutics. 2021. PMID: 35056952 Free PMC article.
-
Carbon monoxide ameliorates lipopolysaccharide-induced acute lung injury via inhibition of alveolar macrophage pyroptosis.Exp Anim. 2023 Feb 21;72(1):77-87. doi: 10.1538/expanim.22-0023. Epub 2022 Oct 3. Exp Anim. 2023. PMID: 36184484 Free PMC article.
References
-
- Bhatia M, Moochhala S. (2004). Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol 202:145–56. - PubMed
-
- Buehler PW, D’Agnillo F, Schaer DJ. (2010). Hemoglobin-based oxygen carriers: From mechanisms of toxicity and clearance to rational drug design. Trends Mol Med 16:447–57. - PubMed
-
- Coburn RF. (1970). The carbon monoxide body stores. Ann NY Acad Sci 174:11–22. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
