Hyperfiltration-mediated Injury in the Remaining Kidney of a Transplant Donor
- PMID: 29847501
- PMCID: PMC6153061
- DOI: 10.1097/TP.0000000000002304
Hyperfiltration-mediated Injury in the Remaining Kidney of a Transplant Donor
Abstract
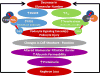
Kidney donors face a small but definite risk of end-stage renal disease 15 to 30 years postdonation. The development of proteinuria, hypertension with gradual decrease in kidney function in the donor after surgical resection of 1 kidney, has been attributed to hyperfiltration. Genetic variations, physiological adaptations, and comorbidities exacerbate the hyperfiltration-induced loss of kidney function in the years after donation. A focus on glomerular hemodynamics and capillary pressure has led to the development of drugs that target the renin-angiotensin-aldosterone system (RAAS), but these agents yield mixed results in transplant recipients and donors. Recent work on glomerular biomechanical forces highlights the differential effects of tensile stress and fluid flow shear stress (FFSS) from hyperfiltration. Capillary wall stretch due to glomerular capillary pressure increases tensile stress on podocyte foot processes that cover the capillary. In parallel, increased flow of the ultrafiltrate due to single-nephron glomerular filtration rate elevates FFSS on the podocyte cell body. Although tensile stress invokes the RAAS, FFSS predominantly activates the cyclooxygenase 2-prostaglandin E2-EP2 receptor axis. Distinguishing these 2 mechanisms is critical, as current therapeutic approaches focus on the RAAS system. A better understanding of the biomechanical forces can lead to novel therapeutic agents to target FFSS through the cyclooxygenase 2-prostaglandin E2-EP2 receptor axis in hyperfiltration-mediated injury. We present an overview of several aspects of the risk to transplant donors and discuss the relevance of FFSS in podocyte injury, loss of glomerular barrier function leading to albuminuria and gradual loss of renal function, and potential therapeutic strategies to mitigate hyperfiltration-mediated injury to the remaining kidney.
Conflict of interest statement
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States Government. The authors declare no conflicts of interest.
Figures


References
-
- O’Keeffe LM, Ramond A, Oliver-Williams C, et al. Mid- and long-term health risks in living kidney donors: a systematic review and meta-analysis. Ann Intern Med. 2018;168:276–284. - PubMed
-
- Boudville N, Prasad GV, Knoll G, et al. Donor nephrectomy outcomes research (DONOR) network. Meta-analysis: risk for hypertension in living kidney donors. Ann Intern Med. 2006;145:185–196. - PubMed
-
- Garg AX, Muirhead N, Knoll G, et al. Donor nephrectomy outcomes research (DONOR) network. Proteinuria and reduced kidney function in living kidney donors: A systematic review, meta-analysis, and meta-regression. Kidney Int. 2006;70:1801–1810. - PubMed
-
- Mjøen G, Hallan S, Hartmann A, et al. Long-term risks for kidney donors. Kidney Int. 2014;86:162–167. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

