Implantable miniature telescope (IMT) for vision loss due to end-stage age-related macular degeneration
- PMID: 29847689
- PMCID: PMC6022289
- DOI: 10.1002/14651858.CD011140.pub2
Implantable miniature telescope (IMT) for vision loss due to end-stage age-related macular degeneration
Abstract
Background: Age-related macular degeneration (AMD) causes progressive and irreversible damage to the retina, resulting in loss of central vision. AMD is the third leading cause of irreversible visual impairment worldwide and the leading cause of blindness in industrialized countries. Since AMD is more common in older individuals, the number of affected individuals will increase significantly as the population ages. The implantable miniature telescope (IMT) is an ophthalmic device developed to improve vision in individuals who have lost vision due to AMD. Once implanted, the IMT is used to enlarge objects in the central visual field and focus them onto healthy areas of the retina not affected by AMD, allowing individuals to recognize objects that they otherwise could not see. It is unclear whether and how much the IMT can improve vision in individuals with end-stage AMD.
Objectives: To assess the effectiveness and safety of the IMT in improving visual acuity and quality of life in people with late or advanced AMD.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Trials Register) (2017, Issue 11); Ovid MEDLINE; Embase.com; PubMed; LILACS; AMED; Web of Science Conference Proceedings Citation Index-Science; OpenSIGLE; the metaRegister of Controlled Trials (mRCT) (last searched 27 June 2014); ClinicalTrials.gov; the ICTRP and the US Food and Drug Administration (FDA) Medical Devices database. The date of the search was 2 November 2017, with the exception of mRCT which is no longer in service.
Selection criteria: We planned to include randomized controlled trials (RCTs) and quasi-randomized trials that compared the IMT versus no IMT.
Data collection and analysis: Two review authors independently assessed all studies for inclusion, using standard methodological procedures expected by Cochrane.
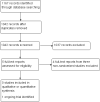
Main results: Our search yielded 1042 unique records. We removed irrelevant studies after screening titles and abstracts, and evaluated five full-text reports from four studies; three were non-randomized studies. There was one ongoing RCT that compared the OriLens intraocular telescope with standard low vision training in eyes with end-stage AMD. Results for this study are expected in 2020.
Authors' conclusions: We found no RCT or quasi-RCT and can draw no conclusion about the effectiveness and safety of the IMT in improving visual acuity in individuals with late or advanced AMD. Since the IMT is typically implanted monocularly based upon which eye has better best-corrected distance visual acuity, randomization between eyes within an individual may not be acceptable. Studies are needed that compare outcomes between individuals randomized to the device versus individuals not implanted, at least during study follow-up, who serve as controls.
Conflict of interest statement
Amisha Gupta: None known. Jessica Lam; None known. Peter Custis: None known. Stephen Munz: None known. Donald Fong: None known. Marguerite Koster: None known.
Update of
- doi: 10.1002/14651858.CD011140
References
References to studies excluded from this review
Brown 2011 {published data only}
-
- Brown GC, Brown MM, Lieske HB, Lieske PA, Brown KS, Lane SS. Comparative effectiveness and cost‐effectiveness of the implantable miniature telescope. Ophthalmology 2011;118(9):1834‐43. - PubMed
Hudson 2006 {published data only}
-
- Hudson HL, Lane SS, Heier JS, Stulting RD, Singerman L, Lichter PR, et al. Implantable miniature telescope for the treatment of visual acuity loss resulting from end‐stage age‐related macular degeneration: 1‐year results. Ophthalmology 2006;113(11):1987‐2001. - PubMed
-
- Hudson HL, Stulting RD, Heier JS, Lane SS, Chang DF, Singerman LJ, et al. Implantable telescope for end‐stage age‐related macular degeneration: long‐term visual acuity and safety outcomes. American Journal of Ophthalmology 2008;146(5):664‐73. - PubMed
Lane 2004 {published data only}
-
- Lane SS, Kuppermann BD, Fine IH, Hamill MB, Gordon JF, Chuck RS, et al. A prospective multicenter clinical trial to evaluate the safety and effectiveness of the implantable miniature telescope. American Journal of Ophthalmology 2004;137(6):993‐1001. - PubMed
References to ongoing studies
MIRROR 2017 {published data only}
-
- How well does the OriLens (Hubble‐type) implant work in improving vision in age‐related macular degeneration?. Available at https://doi.org/10.1186/ISRCTN47403123 (last accessed 14 May 2018).
Additional references
AAO 2014
-
- The Foundation of the American Academy of Ophthalmology. Top 5 Risk Factors for AMD. www.aao.org/eye‐health/news/top‐5‐risk‐factors‐amd (accessed 3 April 2018).
Beck 2007
-
- Beck RW, Maguire MG, Bressler NM, Glassman AR, Lindblad AS, Ferris FL. Visual acuity as an outcome measure in clinical trials of retinal diseases. Ophthalmology 2007;114(10):1804‐9. - PubMed
Bennion 2012
-
- Bennion AE, Shaw RL, Gibson JM. What do we know about the experience of age related macular degeneration? A systematic review and meta‐synthesis of qualitative research. Social Science and Medicine 2012;75(6):976‐85. - PubMed
Deeks 2011
-
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
FDA 2009
-
- FDA. FDA Ophthalmic Devices Advisory Panel information package for March 27, 2009. Review of P050034. VisionCare Ophthalmic Technologies implantable miniature telescope. Volume I of III. www.fda.gov/ohrms/dockets/ac/09/briefing/2009‐4423b1‐04‐Sponsor%20Execut... (accessed 3 April 2018).
FDA 2010
-
- FDA. Summary of safety and effectiveness data, July 2010. PMA P050034. Implantable miniature telescope. www.accessdata.fda.gov/cdrh_docs/pdf5/P050034b.pdf (accessed 3 April 2018).
FDA 2014
-
- FDA. PMA Supplement. Implantable Miniature Telescope Models Wide Angle 2.2X and Wide Angle 2.7X. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma_template.cfm?id=p05... (accessed 3 April 2018).
Ferris 1984
-
- Ferris FL, Fine SL, Hyman L. Age‐related macular degeneration and blindness due to neovascular maculopathy. Archives of Ophthalmology 1984;102(11):1640‐2. - PubMed
Garcia‐Layana 2017
Gehlbach 2016
GRADEpro 2014 [Computer program]
-
- GRADE Working Group, McMaster University. GRADEpro GDT. Version (accessed prior to 10 January 2018). Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Hau 2016
Higgins 2011
-
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Mitchell 2006
National Eye Institute 2012
-
- National Eye Institute. Facts about age‐related macular degeneration. nei.nih.gov/health/maculardegen/armd_facts (accessed 3 April 2018).
Rein 2006
-
- Rein DB, Zhang P, Wirth KE, Lee PP, Hoerger TJ, McCall N, et al. The economic burden of major adult visual disorders in the United States. Archives of Ophthalmology 2006;124(12):1754‐60. - PubMed
Rein 2009
-
- Rein DB, Wittenborn JS, Zhang X, Honeycutt AA, Lesesne SB, Saaddine J, et al. Forecasting age‐related macular degeneration through the year 2050: the potential impact of new treatments. Archives of Ophthalmology 2009;127(4):533‐40. - PubMed
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rudnicka 2012
-
- Rudnicka AR, Jarrar Z, Wormald R, Cook DG, Fletcher A, Owen CG. Age and gender variations in age‐related macular degeneration prevalence in populations of European ancestry: a meta‐analysis. Ophthalmology 2012;119(3):571‐80. - PubMed
SST 2005
-
- Submacular Surgery Trials Research Group. Health‐ and vision‐related quality of life among patients with ocular histoplasmosis or idiopathic choroidal neovascularization at enrollment in a randomized trial of submacular surgery: Submacular Surgery Trials Report No. 5. Archives of Ophthalmology 2005;123(1):78‐88. - PMC - PubMed
Sterne 2011
-
- Sterne JA, Egger M, Moher D, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
VisionCare 2010
-
- VisionCare Ophthalmic Technologies. VisionCare's Implantable Miniature Telescope. An Intraocular Telescope for Treating Severe to Profound Vision Impairment due to Bilateral End‐Stage Age‐Related Macular Degeneration. Patient Information Booklet. VisionCare Ophthalmic Technologies, 2010.
Williams 1998
-
- Williams RA, Brody BL, Thomas RG, Kaplan RM, Brown SI. The psychosocial impact of macular degeneration. Archives of Ophthalmology 1998;116(4):514‐20. - PubMed
World Health Organization 2012
-
- World Health Organization. Prevention of blindness and visual impairment. Priority eye diseases. Age‐related macular degeneration. www.who.int/blindness/causes/priority/en/index7.html (accessed 3 April 2018).
References to other published versions of this review
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical