A Batf3/Nlrp3/IL-18 Axis Promotes Natural Killer Cell IL-10 Production during Listeria monocytogenes Infection
- PMID: 29847790
- PMCID: PMC6170157
- DOI: 10.1016/j.celrep.2018.04.106
A Batf3/Nlrp3/IL-18 Axis Promotes Natural Killer Cell IL-10 Production during Listeria monocytogenes Infection
Abstract
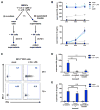
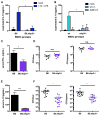
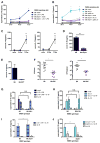
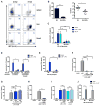
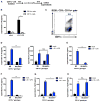
The bacterial pathogen Listeria monocytogenes (Lm) capitalizes on natural killer (NK) cell production of regulatory interleukin (IL)-10 to establish severe systemic infections. Here, we identify regulators of this IL-10 secretion. We show that IL-18 signals to NK cells license their ability to produce IL-10. IL-18 acts independent of IL-12 and STAT4, which co-stimulate IFNγ secretion. Dendritic cell (DC) expression of Nlrp3 is required for IL-18 release in response to the Lm p60 virulence protein. Therefore, mice lacking Nlrp3, Il18, or Il18R fail to accumulate serum IL-10 and are highly resistant to systemic Lm infection. We further show that cells expressing or dependent on Batf3 are required for IL-18-inducing IL-10 production observed in infected mice. These findings explain how Il18 and Batf3 promote susceptibility to bacterial infection and demonstrate the ability of Lm to exploit NLRP3 for the promotion of regulatory NK cell activity.
Keywords: Batf3; Listeria monocytogenes; Natural killer cell; Nlrp3; dendritic cell; immune regulation; infection; innate immunity; interleukin-10; interleukin-18.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures

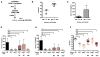
References
-
- Berg RE, Cordes CJ, Forman J. Contribution of CD8+ T cells to innate immunity: IFN-gamma secretion induced by IL-12 and IL-18. Eur J Immunol. 2002;32:2807–2816. - PubMed
-
- Borg C, Jalil A, Laderach D, Maruyama K, Wakasugi H, Charrier S, Ryffel B, Cambi A, Figdor C, Vainchenker W, et al. NK cell activation by dendritic cells (DCs) requires the formation of a synapse leading to IL-12 polarization in DCs. Blood. 2004;104:3267–3275. - PubMed
-
- Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16:407–420. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

