Non-invasive dural stimulation in mice: A novel preclinical model of migraine
- PMID: 29848109
- PMCID: PMC6499065
- DOI: 10.1177/0333102418779557
Non-invasive dural stimulation in mice: A novel preclinical model of migraine
Abstract
Background: Migraine is characterized by a collection of neurological symptoms in the absence of injury or damage. However, several common preclinical migraine models require significant damage to the skull to stimulate the dura mater, the likely source of afferent signaling leading to head pain. The goal of this study was to determine whether dural stimulation can be performed in mice using an injection that does not cause injury or damage.
Methods: Using mice, injections of stimuli were administered to the dura mater through the soft tissue at the intersection between the lambdoidal and sagittal sutures. This technique did not require a permanent cannula nor did it cause damage to the skull or dura. Following injection of noxious stimuli, migraine-like behaviors were measured including cutaneous allodynia and facial grimace. The retrograde tracer fluorogold was applied onto the dura using the same injection technique to label trigeminal ganglion cell bodies, which were then testing in vitro using patch-clamp electrophysiology.
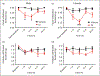
Results: Dural injection of allyl-isothiocyanate, low pH, interleukin-6, or inflammatory soup but not vehicles, led to cephalic/extracephalic allodynia. Facial grimace responses were also observed with allyl-isothiocyanate, pH 6.0, and interleukin-6. Stimulation with interleukin-6 causes priming to normally subthreshold pH 7.0 stimulation of the dura following resolution of the initial interleukin-6 behavior. Systemic injection of sumatriptan at the time of dural stimulation with inflammatory soup decreased the resulting cutaneous hypersensitivity. Trigeminal ganglion cell bodies retrogradely labeled from the dura had low pH-evoked currents similar to those generated by acid-sensing ion channels.
Conclusion: Non-invasive dural stimulation in mice can be used as a model of migraine in the absence of injury.
Keywords: Dura; headache; interleukin; meninges; migraine; mouse model; priming; trigeminal.
Conflict of interest statement
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures



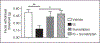

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

