Trigger Tool-Based Automated Adverse Event Detection in Electronic Health Records: Systematic Review
- PMID: 29848467
- PMCID: PMC6000482
- DOI: 10.2196/jmir.9901
Trigger Tool-Based Automated Adverse Event Detection in Electronic Health Records: Systematic Review
Abstract
Background: Adverse events in health care entail substantial burdens to health care systems, institutions, and patients. Retrospective trigger tools are often manually applied to detect AEs, although automated approaches using electronic health records may offer real-time adverse event detection, allowing timely corrective interventions.
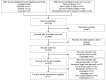
Objective: The aim of this systematic review was to describe current study methods and challenges regarding the use of automatic trigger tool-based adverse event detection methods in electronic health records. In addition, we aimed to appraise the applied studies' designs and to synthesize estimates of adverse event prevalence and diagnostic test accuracy of automatic detection methods using manual trigger tool as a reference standard.
Methods: PubMed, EMBASE, CINAHL, and the Cochrane Library were queried. We included observational studies, applying trigger tools in acute care settings, and excluded studies using nonhospital and outpatient settings. Eligible articles were divided into diagnostic test accuracy studies and prevalence studies. We derived the study prevalence and estimates for the positive predictive value. We assessed bias risks and applicability concerns using Quality Assessment tool for Diagnostic Accuracy Studies-2 (QUADAS-2) for diagnostic test accuracy studies and an in-house developed tool for prevalence studies.
Results: A total of 11 studies met all criteria: 2 concerned diagnostic test accuracy and 9 prevalence. We judged several studies to be at high bias risks for their automated detection method, definition of outcomes, and type of statistical analyses. Across all the 11 studies, adverse event prevalence ranged from 0% to 17.9%, with a median of 0.8%. The positive predictive value of all triggers to detect adverse events ranged from 0% to 100% across studies, with a median of 40%. Some triggers had wide ranging positive predictive value values: (1) in 6 studies, hypoglycemia had a positive predictive value ranging from 15.8% to 60%; (2) in 5 studies, naloxone had a positive predictive value ranging from 20% to 91%; (3) in 4 studies, flumazenil had a positive predictive value ranging from 38.9% to 83.3%; and (4) in 4 studies, protamine had a positive predictive value ranging from 0% to 60%. We were unable to determine the adverse event prevalence, positive predictive value, preventability, and severity in 40.4%, 10.5%, 71.1%, and 68.4% of the studies, respectively. These studies did not report the overall number of records analyzed, triggers, or adverse events; or the studies did not conduct the analysis.
Conclusions: We observed broad interstudy variation in reported adverse event prevalence and positive predictive value. The lack of sufficiently described methods led to difficulties regarding interpretation. To improve quality, we see the need for a set of recommendations to endorse optimal use of research designs and adequate reporting of future adverse event detection studies.
Keywords: electronic health records; patient harm; patient safety; review, systematic.
©Sarah N Musy, Dietmar Ausserhofer, René Schwendimann, Hans Ulrich Rothen, Marie-Madlen Jeitziner, Anne WS Rutjes, Michael Simon. Originally published in the Journal of Medical Internet Research (http://www.jmir.org), 30.05.2018.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures



References
-
- Woloshynowych M, Neale G, Vincent C. Case record review of adverse events: a new approach. Qual Saf Health Care. 2003 Dec;12(6):411–5. http://qhc.bmj.com/cgi/pmidlookup?view=long&pmid=14645755 - PMC - PubMed
-
- Vincent C, Neale G, Woloshynowych M. Adverse events in British hospitals: preliminary retrospective record review. Br Med J. 2001 Mar 3;322(7285):517–9. http://europepmc.org/abstract/MED/11230064 - PMC - PubMed
-
- Institute of Medicine . In: To Err is Human: Building a Safer Health System. Kohn LT, Corrigan JM, Donaldson MS, editors. Washington, DC: The National Academies Press; 2000. - PubMed
-
- Classen D. Medication safety: moving from illusion to reality. J Am Med Assoc. 2003 Mar 05;289(9):1154–6.jed30009 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

