The plant cysteine oxidases from Arabidopsis thaliana are kinetically tailored to act as oxygen sensors
- PMID: 29848548
- PMCID: PMC6066304
- DOI: 10.1074/jbc.RA118.003496
The plant cysteine oxidases from Arabidopsis thaliana are kinetically tailored to act as oxygen sensors
Abstract
Group VII
Keywords: Arabidopsis; ERF-VII; N-end rule; enzyme kinetics; hypoxia; oxygen-sensing; plant biochemistry; plant cysteine oxidase; post-translational modification (PTM); protein degradation.
© 2018 White et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
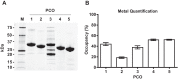
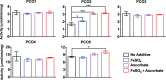
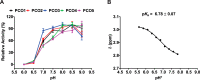
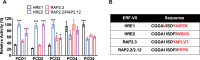
Figures


References
-
- Hattori Y., Nagai K., Furukawa S., Song X. J., Kawano R., Sakakibara H., Wu J., Matsumoto T., Yoshimura A., Kitano H., Matsuoka M., Mori H., and Ashikari M. (2009) The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature 460, 1026–1030 10.1038/nature08258 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

