Efficacy of BGJ398, a Fibroblast Growth Factor Receptor 1-3 Inhibitor, in Patients with Previously Treated Advanced Urothelial Carcinoma with FGFR3 Alterations
- PMID: 29848605
- PMCID: PMC6716598
- DOI: 10.1158/2159-8290.CD-18-0229
Efficacy of BGJ398, a Fibroblast Growth Factor Receptor 1-3 Inhibitor, in Patients with Previously Treated Advanced Urothelial Carcinoma with FGFR3 Alterations
Abstract
BGJ398, a potent and selective pan-FGFR antagonist, was prospectively evaluated in patients with metastatic urothelial carcinoma bearing a diverse array of FGFR3 alterations. Patients (N = 67) who were unable to receive platinum chemotherapy were enrolled. The majority (70.1%) had received two or more prior antineoplastic therapies. BGJ398 was administered orally at 125 mg/day on a 3 weeks on, 1 week off schedule until unacceptable toxicity or progression. The primary endpoint was the response rate. Among 67 patients treated, an overall response rate of 25.4% was observed and an additional 38.8% of patients had disease stabilization, translating to a disease control rate of 64.2%. The most common treatment-emergent toxicities were hyperphosphatemia, elevated creatinine, fatigue, constipation, and decreased appetite. Further examination of BGJ398 in this disease setting is warranted.Significance: BJG398 is active in patients with alterations in FGFR3, resulting in both reductions in tumor volume and stabilization of disease. Our data highlight putative mechanisms of resistance to the agent, which may be useful in following disease status. Cancer Discov; 8(7); 812-21. ©2018 AACR.This article is highlighted in the In This Issue feature, p. 781.
©2018 American Association for Cancer Research.
Conflict of interest statement
Conflict of Interest Disclosure Statement:
Sumanta K. Pal has received consultancy fees from Genentech, Aveo, Eisai, Roche, Pfizer, Novartis, Exelixis, Ipsen, BMS, and Astellas, and honoraria from Genentech. Jonathan E. Rosenberg has received non-financial support from Novartis, grants from Novartis, consultancy fees from Bayer, Lilly, BMS, AstraZeneca, Merck, and EMD-Serono, and grants and consultancy fees from Agensys and Roche/Genentech. Jean H. Hoffman-Censits has received research funding from BioClin Therapeutics, consultancy fees from Foundation Medicine, Genentech and Clovis Oncology, and honoraria from Genentech. David I. Quinn has received consultancy fees from Merck, BMS, Genentech, Roche, AstraZeneca, EMD Serono, Pfizer, and Bayer. Matthew D. Galsky has received consultancy fees from Genentech, Merck, Novartis, Astellas, AstraZeneca, and BMS, and has stock with Dual Therapeutics. Juergen Wolf has received personal fees from AbbVie, AstraZeneca, BMS, Boehringer Ingelheim, Chugai, Ignyta, Eli Lilly, Merck, Novartis, Pfizer, and Roche, and grants from BMS, Merck, Novartis, and Pfizer. Christian Dittrich has received personal fees from Novartis, AstraZeneca, Eli Lilly, Merck Serono, Roche, Sanofi, Bristol-Myers Squibb, and Ipsen, grants from Merck, Amgen, Pierre Fabre, Eisai, Bayer, AstraZeneca, Boehringer Ingelheim, Pfizer, Roche, Novartis, and Sanofi, and non-financial support from Bayer, Takeda, Sanofi, Merck, and Servier. Virote Sriuranpong has received honoraria from Novartis. Viktor Grünwald has received grants, personal fees and non-financial support from BMS and MSD, personal fees and non-financial support from Roche, Novartis, and Ipsen, grants and personal fees from Pfizer and AstraZeneca, and personal fees from Eisai, EUSA Pharma, and Cerulean. Daniel Petrylak has received personal fees from Bayer, Bellicum, Dendreon, Exelixis, Ferring, Johnson and Johnson, Medivation, Millennium, Pfizer, Roche Laboratories, Sanofi Aventis, and Tyme Pharmaceuticals, grants related to this work from Novartis, grants not related to this work from Agensys, AstraZeneca, Bayer, Dendreon, Eli Lilly, Endocyte, Genentech, Innocrin, Johnson and Johnson, MedImmune, Medivation, Merck, Millennium, OncoGenex, Pfizer, Progenics, Roche Laboratories, Sanofi Aventis, and Sotio, owns stock with Bellicum and Tyme, and has a patent pending relevant to this work. Ulka Vaishampayan has received grants and personal fees from Novartis, Bristol Myers Squibb, Exelixis, Pfizer, and Astellas, and personal fees from Bayer and Sanofi. Eliahu Gez has received funding from Sourasky Medical Centre, related to this work. Ugo De Giorgi has received personal fees from Pierre-Fabre, Sanofi, Astellas, Novartis, and Ipsen, and personal fees and non-financial support from Janssen and BMS. Amir Mortazavi has received consultancy fees from Genentech-Roche; and his institution, The Ohio State University, has received grants from Acerta Pharma, Genentech, Merck, and Novartis for clinical trials operation. Katie Parker is a former employee of Novartis and owns Novartis stock. Dean F. Bajorin has received research support and has acted as a consultant for Novartis, Merck, Genentech and Pfizer; received honorari from Merck, and has been a consultant for Urogen and Fidia Farmaceutici. Kun Yu is an employee of Novartis. Xueying Chen, Diana Graus Porta and Randi Isaacs are employees of Novartis and own Novartis stock. Dale Porter is an employee of Novartis, owns Novartis stock, and has a patent pending for BGJ398 drug combinations. Ranaan Berger, Gwenaelle Gravis, Jacques Medioni, Howard A. Burris, Bhumsuk Keam, Jean-Pierre Delord, David J. Vaughn, Jens Voortman, Sumati Gupta, Chaiyut Charoentum, Pablo Maroto, Jan H. M. Schellens, and Jae-Lyun Lee have no competing financial interests.
Figures
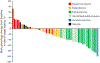
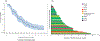

References
-
- von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 2000;18(17):3068–77. - PubMed
-
- Sonpavde G, Sternberg CN, Rosenberg JE, Hahn NM, Galsky MD, Vogelzang NJ. Second-line systemic therapy and emerging drugs for metastatic transitional-cell carcinoma of the urothelium. Lancet Oncol 2010;11(9):861–70. - PubMed
-
- Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016;387(10031):1909–20. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

