A theta rhythm in macaque visual cortex and its attentional modulation
- PMID: 29848632
- PMCID: PMC6004461
- DOI: 10.1073/pnas.1719433115
A theta rhythm in macaque visual cortex and its attentional modulation
Abstract
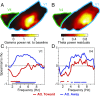
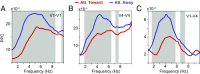
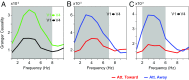
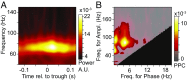
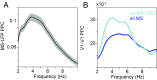
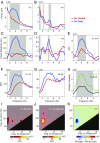
Theta rhythms govern rodent sniffing and whisking, and human language processing. Human psychophysics suggests a role for theta also in visual attention. However, little is known about theta in visual areas and its attentional modulation. We used electrocorticography (ECoG) to record local field potentials (LFPs) simultaneously from areas V1, V2, V4, and TEO of two macaque monkeys performing a selective visual attention task. We found a ≈4-Hz theta rhythm within both the V1-V2 and the V4-TEO region, and theta synchronization between them, with a predominantly feedforward directed influence. ECoG coverage of large parts of these regions revealed a surprising spatial correspondence between theta and visually induced gamma. Furthermore, gamma power was modulated with theta phase. Selective attention to the respective visual stimulus strongly reduced these theta-rhythmic processes, leading to an unusually strong attention effect for V1. Microsaccades (MSs) were partly locked to theta. However, neuronal theta rhythms tended to be even more pronounced for epochs devoid of MSs. Thus, we find an MS-independent theta rhythm specific to visually driven parts of V1-V2, which rhythmically modulates local gamma and entrains V4-TEO, and which is strongly reduced by attention. We propose that the less theta-rhythmic and thereby more continuous processing of the attended stimulus serves the exploitation of this behaviorally most relevant information. The theta-rhythmic and thereby intermittent processing of the unattended stimulus likely reflects the ecologically important exploration of less relevant sources of information.
Keywords: attention; gamma; phase-amplitude coupling; theta; visual cortex.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Frequency-dependent spatiotemporal profiles of visual responses recorded with subdural ECoG electrodes in awake monkeys: Differences between high- and low-frequency activity.Neuroimage. 2016 Jan 1;124(Pt A):557-572. doi: 10.1016/j.neuroimage.2015.09.007. Epub 2015 Sep 10. Neuroimage. 2016. PMID: 26363347
-
Attention Configures Synchronization Within Local Neuronal Networks for Processing of the Behaviorally Relevant Stimulus.Front Neural Circuits. 2018 Aug 29;12:71. doi: 10.3389/fncir.2018.00071. eCollection 2018. Front Neural Circuits. 2018. PMID: 30210309 Free PMC article.
-
Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex.J Neurophysiol. 1997 Jan;77(1):24-42. doi: 10.1152/jn.1997.77.1.24. J Neurophysiol. 1997. PMID: 9120566
-
The neural bases of spatial attention and perceptual rhythms.Eur J Neurosci. 2022 Jun;55(11-12):3209-3223. doi: 10.1111/ejn.15044. Epub 2020 Nov 27. Eur J Neurosci. 2022. PMID: 33185294 Review.
-
A Rhythmic Theory of Attention.Trends Cogn Sci. 2019 Feb;23(2):87-101. doi: 10.1016/j.tics.2018.11.009. Epub 2018 Dec 24. Trends Cogn Sci. 2019. PMID: 30591373 Free PMC article. Review.
Cited by
-
An Attempt at a Unified Theory of the Neocortical Microcircuit in Sensory Cortex.Front Neural Circuits. 2020 Jul 28;14:40. doi: 10.3389/fncir.2020.00040. eCollection 2020. Front Neural Circuits. 2020. PMID: 32848632 Free PMC article.
-
Contribution of FEF to Attentional Periodicity during Visual Search: A TMS Study.eNeuro. 2019 Jun 24;6(3):ENEURO.0357-18.2019. doi: 10.1523/ENEURO.0357-18.2019. Print 2019 May/Jun. eNeuro. 2019. PMID: 31175148 Free PMC article.
-
Effects of high-frequency transcranial magnetic stimulation on theta-gamma oscillations and coupling in the prefrontal cortex of rats during working memory task.Med Biol Eng Comput. 2023 Dec;61(12):3209-3223. doi: 10.1007/s11517-023-02940-w. Epub 2023 Oct 13. Med Biol Eng Comput. 2023. PMID: 37828414
-
Decoding Adaptive Visuomotor Behavior Mediated by Non-linear Phase Coupling in Macaque Area MT.Front Neurosci. 2020 Apr 3;14:230. doi: 10.3389/fnins.2020.00230. eCollection 2020. Front Neurosci. 2020. PMID: 32317912 Free PMC article.
-
Revisiting Persistent Neuronal Activity During Covert Spatial Attention.Front Neural Circuits. 2021 Jun 30;15:679796. doi: 10.3389/fncir.2021.679796. eCollection 2021. Front Neural Circuits. 2021. PMID: 34276314 Free PMC article. Review.
References
-
- O’Keefe J, Recce ML. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3:317–330. - PubMed
-
- Buzsáki G. 2006. Rhythms of the Brain (Oxford Univ Press, Oxford), p xiv, 448 p.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

