Antimicrobial and Chemotactic Activity of Scorpion-Derived Peptide, ToAP2, against Mycobacterium massiliensis
- PMID: 29848960
- PMCID: PMC6024781
- DOI: 10.3390/toxins10060219
Antimicrobial and Chemotactic Activity of Scorpion-Derived Peptide, ToAP2, against Mycobacterium massiliensis
Abstract
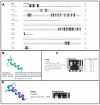
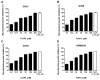
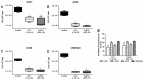
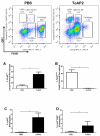
Mycobacterium massiliense is a rapid growing, multidrug-resistant, non-tuberculous mycobacteria that is responsible for a wide spectrum of skin and soft tissue infections, as well as other organs, such as the lungs. Antimicrobial peptides had been described as broad-spectrum antimicrobial, chemotactic, and immunomodulator molecules. In this study we evaluated an antimicrobial peptide derived from scorpion Tityus obscurus as an anti-mycobacterial agent in vitro and in vivo. Bioinformatics analyses demonstrated that the peptide ToAP2 have a conserved region similar to several membrane proteins, as well as mouse cathelicidin. ToAP2 inhibited the growth of four M. massiliense strains (GO01, GO06, GO08, and CRM0020) at a minimal bactericidal concentration (MBC) of 200 µM. MBC concentration used to treat infected macrophages was able to inhibit 50% of the bacterial growth of all strains. ToAP2 treatment of infected mice with bacilli reduced the bacterial load in the liver, lung, and spleen, similarly to clarithromycin levels (90%). ToAP2 alone recruited monocytes (F4/80low Gr1), neutrophils (F4/80- Gr1), and eosinophils (F4/80+ Gr1+). ToAP2, together with M. massiliense infection, was able to increase F4/80low and reduce the percentage of F4/80high macrophages when compared with infected and untreated mice. ToAP2 has in vitro anti-microbial activity that is improved in vivo due to chemotactic activity.
Keywords: Mycobacterium; antimicrobial peptide; monocyte; mycobacteria inhibition; neutrophil.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Antimycobacterial Activity of a New Peptide Polydim-I Isolated from Neotropical Social Wasp Polybia dimorpha.PLoS One. 2016 Mar 1;11(3):e0149729. doi: 10.1371/journal.pone.0149729. eCollection 2016. PLoS One. 2016. PMID: 26930596 Free PMC article.
-
GM-CSF knockout mice for preclinical testing of agents with antimicrobial activity against Mycobacterium abscessus.J Antimicrob Chemother. 2014 Apr;69(4):1057-64. doi: 10.1093/jac/dkt451. Epub 2013 Nov 11. J Antimicrob Chemother. 2014. PMID: 24222613
-
Non-disulfide-Bridge Peptide 5.5 from the Scorpion Hadrurus gertschi Inhibits the Growth of Mycobacterium abscessus subsp. massiliense.Front Microbiol. 2017 Feb 22;8:273. doi: 10.3389/fmicb.2017.00273. eCollection 2017. Front Microbiol. 2017. PMID: 28275372 Free PMC article.
-
Experimental chemotherapy of mycobacterioses provoked by atypical mycobacteria.Bibl Tuberc. 1970;26:154-88. Bibl Tuberc. 1970. PMID: 4983240 Review. No abstract available.
-
Infections caused by Mycobacterium abscessus: epidemiology, diagnostic tools and treatment.Expert Rev Anti Infect Ther. 2016 Dec;14(12):1139-1154. doi: 10.1080/14787210.2016.1238304. Epub 2016 Oct 3. Expert Rev Anti Infect Ther. 2016. PMID: 27690688 Review.
Cited by
-
Peptides with Diverse Functions from Scorpion Venom: A Great Opportunity for the Treatment of a Wide Variety of Diseases.Iran Biomed J. 2023 Mar 1;27(2 & 3):84-99. doi: 10.61186/ibj.3863. Iran Biomed J. 2023. PMID: 37070616 Free PMC article.
-
Novel Synthetic Peptide Agelaia-12 Has Improved Activity Against Mycobacterium abscessus Complex.Pathogens. 2024 Nov 13;13(11):994. doi: 10.3390/pathogens13110994. Pathogens. 2024. PMID: 39599547 Free PMC article.
-
Exploring the potential of Brazilian Amazonian scorpion venoms: A comprehensive review of research from 2001 to 2021.Toxicon X. 2023 Dec 29;21:100182. doi: 10.1016/j.toxcx.2023.100182. eCollection 2024 Mar. Toxicon X. 2023. PMID: 38226138 Free PMC article. Review.
-
Assessment of in vitro activities of novel modified antimicrobial peptides against clarithromycin resistant Mycobacterium abscessus.PLoS One. 2021 Nov 15;16(11):e0260003. doi: 10.1371/journal.pone.0260003. eCollection 2021. PLoS One. 2021. PMID: 34780520 Free PMC article.
-
Scorpion Venom: Detriments and Benefits.Biomedicines. 2020 May 12;8(5):118. doi: 10.3390/biomedicines8050118. Biomedicines. 2020. PMID: 32408604 Free PMC article. Review.
References
-
- Cardoso A.M., Martins de Sousa E., Viana-Niero C., Bonfim de Bortoli F., Pereira das Neves Z.C., Leao S.C., Junqueira-Kipnis A.P., Kipnis A. Emergence of Nosocomial Mycobacterium massiliense Infection in Goias, Brazil. Microbes Infect. 2008;10:1552–1557. doi: 10.1016/j.micinf.2008.09.008. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

