Synthesis, Crystal Structure, and Biological Activity of Ethyl 4-Methyl-2,2-dioxo-1 H-2λ⁶,1-benzothiazine-3-carboxylate Polymorphic Forms
- PMID: 29848976
- PMCID: PMC6027672
- DOI: 10.3390/scipharm86020021
Synthesis, Crystal Structure, and Biological Activity of Ethyl 4-Methyl-2,2-dioxo-1 H-2λ⁶,1-benzothiazine-3-carboxylate Polymorphic Forms
Abstract
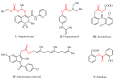
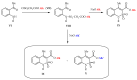
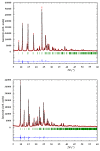
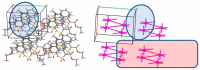
Continuing the search for new potential analgesics among the derivatives of 4-methyl-2,2-dioxo-1H-2λ⁶,1-benzothiazine-3-carboxylic acid, the possibility of obtaining its esters by the alkylation of the corresponding sodium salt with iodoethane in dimethyl sulfoxide (DMSO) at room temperature was studied. It was found that under such conditions, together with the oxygen atom of the carboxyl group, a heteroatom of nitrogen is also alkylated. Therefore, the product of the reaction studied is a mixture of ethyl 4-methyl-2,2-dioxo-1H-2λ⁶,1-benzothiazine-3-carboxylate (major) and its 1-ethyl-substituted analog (minor). A simple but very effective method of preparative separation of these compounds was proposed. Moreover, the heterogeneous crystallization from ethanol was revealed to result in a monoclinic polymorphic form of ethyl 4-methyl-2,2-dioxo-1H-2λ⁶,1-benzothiazine-3-carboxylate, while the homogeneous crystallization results in its orthorhombic form. The molecular and crystal structures of both forms were confirmed by X-ray diffraction analysis, and the phase purity by powder diffraction study. The pharmacological tests carried out on the model of a carrageenan edema showed that the screening dose of 20 mg/kg of 1-ethyl-substituted ester and the orthorhombic form of its analog unsubstituted in position 1 exhibited weak anti-inflammatory and moderate analgesic effects. At the same time, the monoclinic form of ethyl 4-methyl-2,2-dioxo-1H-2λ⁶,1-benzothiazine-3-carboxylate appeared to be both a powerful analgesic and an anti-inflammatory agent that exceeded Piroxicam and Meloxicam in the same doses by these indicators. A detailed comparative analysis of the molecular and crystal structures of two polymorphic forms of ethyl 4-methyl-2,2-dioxo-1H-2λ⁶,1-benzothiazine-3-carboxylate was carried out using quantum chemical calculations of the energies of pairwise interactions between molecules. An explanation of the essential differences of their biological properties based on this was offered.
Keywords: 2,1-benzothiazine; 4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylic acid; alkylation; analgesic activity; anti-inflammatory action; crystal structure; esters; polymorphism.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Kleemann A., Engel J., Kutscher B., Reichert D. Pharmaceutical Substances: Syntheses, Patents, Applications of the Most Relevant APIs. 5th ed. Thieme; Stuttgart, Germany: 2008.
-
- O’Neil M.J., Heckelman P.E., Koch C.B., Roman K.J. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals. 14th ed. Merck and Co., Inc.; Whitehouse Station, NJ, USA: 2006.
-
- Kuznetsov S.G., Chigareva S.M., Ramsh S.M. Prodrugs: Chemical aspect. Results Sci. Technol. VINITI Ser. Org. Chem. 1981;19:1–176.
LinkOut - more resources
Full Text Sources
Other Literature Sources
