Deciphering the cells of origin of squamous cell carcinomas
- PMID: 29849070
- PMCID: PMC7170720
- DOI: 10.1038/s41568-018-0024-5
Deciphering the cells of origin of squamous cell carcinomas
Abstract
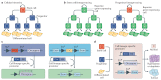
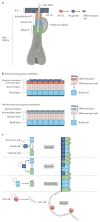
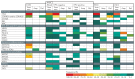
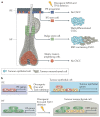
Squamous cell carcinomas (SCCs) are among the most prevalent human cancers. SCC comprises a wide range of tumours originated from diverse anatomical locations that share common genetic mutations and expression of squamous differentiation markers. SCCs arise from squamous and non-squamous epithelial tissues. Here, we discuss the different studies in which the cell of origin of SCCs has been uncovered by expressing oncogenes and/or deleting tumour suppressor genes in the different cell lineages that compose these epithelia. We present evidence showing that the squamous differentiation phenotype of the tumour depends on the type of mutated oncogene and the cell of origin, which dictate the competence of the cells to initiate SCC formation, as well as on the aggressiveness and invasive properties of these tumours.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001;344:975–983. - PubMed
-
- Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9–22. - PubMed
-
- Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med. 2014;371:2499–2509. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

