Fermentative Spirochaetes mediate necromass recycling in anoxic hydrocarbon-contaminated habitats
- PMID: 29849169
- PMCID: PMC6052044
- DOI: 10.1038/s41396-018-0148-3
Fermentative Spirochaetes mediate necromass recycling in anoxic hydrocarbon-contaminated habitats
Abstract
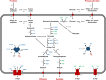
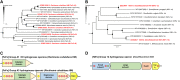
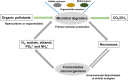
Spirochaetes are frequently detected in anoxic hydrocarbon- and organohalide-polluted groundwater, but their role in such ecosystems has remained unclear. To address this, we studied a sulfate-reducing, naphthalene-degrading enrichment culture, mainly comprising the sulfate reducer Desulfobacterium N47 and the rod-shaped Spirochete Rectinema cohabitans HM. Genome sequencing and proteome analysis suggested that the Spirochete is an obligate fermenter that catabolizes proteins and carbohydrates, resulting in acetate, ethanol, and molecular hydrogen (H2) production. Physiological experiments inferred that hydrogen is an important link between the two bacteria in the enrichment culture, with H2 derived from fermentation by R. cohabitans used as reductant for sulfate reduction by Desulfobacterium N47. Differential proteomics and physiological experiments showed that R. cohabitans utilizes biomass (proteins and carbohydrates) released from dead cells of Desulfobacterium N47. Further comparative and community genome analyses indicated that other Rectinema phylotypes are widespread in contaminated environments and may perform a hydrogenogenic fermentative lifestyle similar to R. cohabitans. Together, these findings indicate that environmental Spirochaetes scavenge detrital biomass and in turn drive necromass recycling at anoxic hydrocarbon-contaminated sites and potentially other habitats.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- Karami A, Sarshar M, Ranjbar R, Zanjani RS. The phylum Spirochaetaceae. In: Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F, editors. The prokaryotes: other major lineages of bacteria and the archaea. Berlin, Heidelberg: Springer Berlin Heidelberg; 2014. pp. 915–29.
-
- Einsiedl F, Pilloni G, Ruth-Anneser B, Lueders T, Griebler C. Spatial distributions of sulphur species and sulphate-reducing bacteria provide insights into sulphur redox cycling and biodegradation hot-spots in a hydrocarbon-contaminated aquifer. Geochim Cosmochim Acta. 2015;156:207–21. doi: 10.1016/j.gca.2015.01.014. - DOI
-
- Selesi D, Jehmlich N, von Bergen M, Schmidt F, Rattei T, Tischler P, et al. Combined genomic and proteomic approaches identify gene clusters involved in anaerobic 2-methylnaphthalene degradation in the sulfate-reducing enrichment culture N47. J Bacteriol. 2010;192:295–306. doi: 10.1128/JB.00874-09. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

