Anti-factor Xa levels in obese patients receiving enoxaparin for treatment and prophylaxis indications
- PMID: 29849468
- PMCID: PMC5965377
- DOI: 10.2147/CPAA.S161599
Anti-factor Xa levels in obese patients receiving enoxaparin for treatment and prophylaxis indications
Abstract
Objectives: To evaluate the degree of anticoagulation achieved with different enoxaparin dosing regimens used in obese and morbidly obese patients in a hospital setting in Jordan.
Methods: All obese adult patients who were prescribed enoxaparin for various indications were invited to participate in the study. The anti-factor Xa (anti-Xa) level was checked once after 4-6 hours of the third or fourth dose of enoxaparin (at steady state). Patients were followed daily to evaluate drug efficacy and safety through their hospital course.
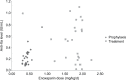
Results: Enoxaparin daily dose used for prophylaxis indications ranged from 0.3 to 0.85 mg/kg and from 0.31 to 2.25 mg/kg in case of certain treatment indications. Most participants who received enoxaparin for treatment indications (76.9%) were on capping dosing regimens, which was <1 mg/kg twice daily. On the other hand, most patients (88.5%) who received enoxaparin for prophylaxis indications were on a fixed 40 mg/d dose. Among the 52 patients who completed the study, 19 patients (36.5%) had therapeutic anti-Xa levels. The results showed no statistically significant associations between regimens that were used and achieving therapeutic anti-Xa level (p>0.05). No bleeding events or thrombocytopenia were noticed, and there was one case of recurrent thrombosis.
Conclusion: Enoxaparin dosing regimens that were used for obese patients varied based on prescribing physicians. Regardless of the regimen used, the majority of participants had nontherapeutic anti-Xa. Individualized dosing regimens based on anti-Xa levels are warranted for obese patients on enoxaparin.
Keywords: anti-factor Xa; anticoagulation; enoxaparin; obesity.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures

References
-
- DiPiro JT, Talbert RL, Yee GC, et al. Pharmacotherapy: A Pathophysiologic Approach. 9th ed. New York, NY: Mcgraw Hill; Education: 2014.
-
- Hirsh J, Bauer KA, Donati MB, Gould M, Samama MM, Weitz JI. Parenteral anticoagulants: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition) Chest. 2008;133(Suppl 6):141S–159S. - PubMed
-
- World Health Organization . Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation. Geneva: World Health Organization; 2000. (WHO Technical Report Series 894). - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

