Diabetes Downregulates Allergen-Induced Airway Inflammation in Mice
- PMID: 29849493
- PMCID: PMC5925213
- DOI: 10.1155/2018/6150843
Diabetes Downregulates Allergen-Induced Airway Inflammation in Mice
Abstract
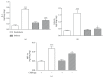
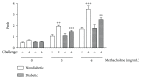
Previous studies described that allergic diseases, including asthma, occur less often than expected in patients with type 1 diabetes. Here, we investigated the influence of diabetes on allergic airway inflammation in a model of experimental asthma in mice. Diabetes was induced by intravenous injection of alloxan into 12 h-fasted A/J mice, followed by subcutaneous sensitization with ovalbumin (OVA) and aluminum hydroxide (Al(OH)3), on days 5 and 19 after diabetes induction. Animals were intranasally challenged with OVA (25 μg), from day 24 to day 26. Alloxan-induced diabetes significantly attenuated airway inflammation as attested by the lower number of total leukocytes in the bronchoalveolar lavage fluid, mainly neutrophils and eosinophils. Suppression of eosinophil infiltration in the peribronchiolar space and generation of eosinophilotactic mediators, such as CCL-11/eotaxin, CCL-3/MIP-1α, and IL-5, were noted in the lungs of diabetic sensitized mice. In parallel, reduction of airway hyperreactivity (AHR) to methacholine, mucus production, and serum IgE levels was also noted under diabetic conditions. Our findings show that alloxan diabetes caused attenuation of lung allergic inflammatory response in A/J mice, by a mechanism possibly associated with downregulation of IgE antibody production.
Figures




References
-
- Spiro S., Silvestri G., Agustí A., editors. Pavord. Clinical Respiratory Medicine. Fourth. Philadelphia, PA, USA: Elsevier; 2012. p. p. 1000.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

