Intranasal Pharmacokinetics of Morphine ARER, a Novel Abuse-Deterrent Formulation: Results from a Randomized, Double-Blind, Four-Way Crossover Study in Nondependent, Opioid-Experienced Subjects
- PMID: 29849845
- PMCID: PMC5937443
- DOI: 10.1155/2018/7276021
Intranasal Pharmacokinetics of Morphine ARER, a Novel Abuse-Deterrent Formulation: Results from a Randomized, Double-Blind, Four-Way Crossover Study in Nondependent, Opioid-Experienced Subjects
Abstract
Objective: To investigate the pharmacokinetics (PK) of Morphine ARER, an extended-release (ER), abuse-deterrent formulation of morphine sulfate after oral and intranasal administration.
Methods: This randomized, double-blind, double-dummy, placebo-controlled, four-way crossover study assessed the PK of morphine and its active metabolite, M6G, from crushed intranasal Morphine ARER and intact oral Morphine ARER compared with crushed intranasal ER morphine following administration to nondependent, recreational opioid users. The correlation between morphine PK and the pharmacodynamic parameter of drug liking, a measure of abuse potential, was also evaluated.
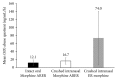
Results: Mean maximum observed plasma concentration (Cmax) for morphine was lower with crushed intranasal Morphine ARER (26.2 ng/mL) and intact oral Morphine ARER (18.6 ng/mL), compared with crushed intranasal ER morphine (49.5 ng/mL). The time to Cmax (Tmax) was the same for intact oral and crushed intranasal Morphine ARER (1.6 hours) and longer for crushed intranasal morphine ER (1.1 hours). Higher mean maximum morphine Cmax, Tmax, and abuse quotient (Cmax/Tmax) were positively correlated with maximum effect for drug liking (R2 ≥ 0.9795).
Conclusion: These data suggest that Morphine ARER maintains its ER profile despite physical manipulation and intranasal administration, which may be predictive of a lower intranasal abuse potential compared with ER morphine.
Figures



References
-
- Center for Behavioral Health Statistics and Quality. Behavioral Health Trends in the United States: Results from the 2014 National Survey on Drug Use and Health. 2015.
-
- Centers for Disease Control and Prevention. Opioid Data Analysis. October 2017.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

