Short-Duration Swimming Exercise after Myocardial Infarction Attenuates Cardiac Dysfunction and Regulates Mitochondrial Quality Control in Aged Mice
- PMID: 29849892
- PMCID: PMC5925211
- DOI: 10.1155/2018/4079041
Short-Duration Swimming Exercise after Myocardial Infarction Attenuates Cardiac Dysfunction and Regulates Mitochondrial Quality Control in Aged Mice
Abstract
Background: Exercise benefits to cardiac rehabilitation (CR) following stable myocardial infarction (MI). The suitable exercise duration for aged patients with coronary heart disease (CHD) remains controversial, and the underlying molecular mechanism is still unclear.
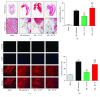
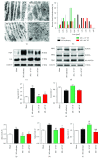
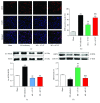
Methods and results: 18-Month-old mice after stable MI were randomly submitted to different durations of exercise, including 15 and 60 min swimming training (ST) once per day, five times a week for 8 weeks. Compared to sedentary mice, 15 min ST, rather than 60 min ST, significantly augmented left ventricular function, increased survival rate, and suppressed myocardial fibrosis and apoptosis. 15 min ST improved mitochondrial morphology via regulating mitochondrial fission-fusion signaling. 15 min ST regulated mitophagy signaling via inhibiting LC3-II and P62 levels and increasing PINK/Parkin expression. 15 min ST also inhibited ROS production and enhanced antioxidant SOD2 activity. Notably, 15 min ST significantly increased sirtuin (SIRT) 3 level (2.7-fold) in vivo while the inhibition of SIRT3 exacerbated senescent H9c2 cellular LDH release and ROS production under hypoxia. In addition, SIRT3 silencing impairs mitochondrial dynamics and mitophagy in senescent cardiomyocytes against simulated ischemia (SI) injury.
Conclusion: Collectively, our study demonstrated for the first time that sustained short-duration exercise, rather than long-duration exercise, attenuates cardiac dysfunction after MI in aged mice. It is likely that the positive regulation induced by a short-duration ST regimen on the elevated SIRT3 protein level improved mitochondrial quality control and decreased apoptosis and fibrosis contributed to the observed more resistant phenotype.
Figures





Similar articles
-
Tetrahydrocurcumin ameliorates postinfarction cardiac dysfunction and remodeling by inhibiting oxidative stress and preserving mitochondrial function via SIRT3 signaling pathway.Phytomedicine. 2023 Dec;121:155127. doi: 10.1016/j.phymed.2023.155127. Epub 2023 Oct 2. Phytomedicine. 2023. PMID: 37812853
-
Polydatin protects cardiomyocytes against myocardial infarction injury by activating Sirt3.Biochim Biophys Acta Mol Basis Dis. 2017 Aug;1863(8):1962-1972. doi: 10.1016/j.bbadis.2016.09.003. Epub 2016 Sep 7. Biochim Biophys Acta Mol Basis Dis. 2017. PMID: 27613967
-
Postinfarction exercise training alleviates cardiac dysfunction and adverse remodeling via mitochondrial biogenesis and SIRT1/PGC-1α/PI3K/Akt signaling.J Cell Physiol. 2019 Dec;234(12):23705-23718. doi: 10.1002/jcp.28939. Epub 2019 Jun 11. J Cell Physiol. 2019. PMID: 31187505
-
Sirt3 deficiency exacerbates diabetic cardiac dysfunction: Role of Foxo3A-Parkin-mediated mitophagy.Biochim Biophys Acta Mol Basis Dis. 2017 Aug;1863(8):1973-1983. doi: 10.1016/j.bbadis.2016.10.021. Epub 2016 Oct 26. Biochim Biophys Acta Mol Basis Dis. 2017. PMID: 27794418 Review.
-
Mitochondrial quality surveillance as a therapeutic target in myocardial infarction.Acta Physiol (Oxf). 2021 Mar;231(3):e13590. doi: 10.1111/apha.13590. Epub 2020 Dec 16. Acta Physiol (Oxf). 2021. PMID: 33270362 Review.
Cited by
-
Exercise: a molecular tool to boost muscle growth and mitochondrial performance in heart failure?Eur J Heart Fail. 2022 Feb;24(2):287-298. doi: 10.1002/ejhf.2407. Epub 2022 Jan 9. Eur J Heart Fail. 2022. PMID: 34957643 Free PMC article. Review.
-
Mitochondrial Quality Control in Cardiomyocytes: A Critical Role in the Progression of Cardiovascular Diseases.Front Physiol. 2020 Mar 27;11:252. doi: 10.3389/fphys.2020.00252. eCollection 2020. Front Physiol. 2020. PMID: 32292354 Free PMC article. Review.
-
Preserving mitochondrial function by inhibiting GRP75 ameliorates neuron injury under ischemic stroke.Mol Med Rep. 2022 May;25(5):165. doi: 10.3892/mmr.2022.12681. Epub 2022 Mar 16. Mol Med Rep. 2022. PMID: 35293600 Free PMC article.
-
Exercise Alleviates Cardiovascular Diseases by Improving Mitochondrial Homeostasis.J Am Heart Assoc. 2024 Oct;13(19):e036555. doi: 10.1161/JAHA.124.036555. Epub 2024 Sep 18. J Am Heart Assoc. 2024. PMID: 39291488 Free PMC article. Review.
-
Mitochondrial dysfunction in cardiovascular disease: Towards exercise regulation of mitochondrial function.Front Physiol. 2023 Jan 19;14:1063556. doi: 10.3389/fphys.2023.1063556. eCollection 2023. Front Physiol. 2023. PMID: 36744035 Free PMC article.
References
-
- Thompson P. D., Buchner D., Pina I. L., et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the council on clinical cardiology (subcommittee on exercise, rehabilitation, and prevention) and the council on nutrition, physical activity, and metabolism (subcommittee on physical activity) Circulation. 2003;107(24):3109–3116. doi: 10.1161/01.CIR.0000075572.40158.77. - DOI - PubMed
-
- Acanfora D., Scicchitano P., Casucci G., et al. Exercise training effects on elderly and middle-age patients with chronic heart failure after acute decompensation: a randomized, controlled trial. International Journal of Cardiology. 2016;225:313–323. doi: 10.1016/j.ijcard.2016.10.026. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

