Lamotrigine ethanol monosolvate
- PMID: 29850090
- PMCID: PMC5947486
- DOI: 10.1107/S2056989018005819
Lamotrigine ethanol monosolvate
Abstract
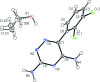
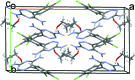
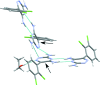
Lamotrigine is an active pharmaceutical ingredient used as a treatment for epilepsy and psychiatric disorders. Single crystals of an ethano-late solvate, C9H7Cl2N5·C2H5OH, were produced by slow evaporation of a saturated solution from anhydrous ethanol. Within the crystal structure, the lamotrigine mol-ecules form dimers through N-H⋯N hydrogen bonds involving the amine N atoms in the ortho position of the triazine group. These dimers are linked into a tape motif through hydrogen bonds involving the amine N atoms in the para position. The ethanol and lamotrigine are present in a 1:1 ratio in the lattice with the ethyl group of the ethanol mol-ecule exhibiting disorder with an occupancy ratio of 0.516 (14):0.484 (14).
Keywords: crystal structure; ethanolate; lamotrigine.
Figures
References
-
- Bruker (2015). APEX2, SAINT and SADABS. Bruker AXS Inc., Madison, Wisconsin USA.
-
- Cheney, M. L., Shan, N., Healey, E. R., Hanna, M., Wojtas, L., Zaworotko, M. J., Sava, V., Song, S. & Sanchez-Ramos, J. R. (2010). Cryst. Growth Des. 10, 394–405.
-
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
-
- Garti, N., Berkovich, Y., Dolitzky, B. Z., Aronhime, J., Singer, C., Liebermann, A. & Gershon, N. (2008). US Patent Number. 7390807B2.
LinkOut - more resources
Full Text Sources
Other Literature Sources
