Acute aortic syndromes and aortic emergencies
- PMID: 29850421
- PMCID: PMC5949592
- DOI: 10.21037/cdt.2018.03.02
Acute aortic syndromes and aortic emergencies
Abstract
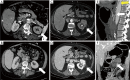
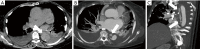
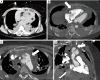
Acute aortic syndrome (AAS) and emergencies are relatively uncommon but are considered as life threatening, potentially fatal conditions. Different forms of aortic emergencies/AAS are often clinically indiscernible. Prompt and accurate diagnosis of these entities significantly influences prognosis and guides therapy. We aim to elucidate the pertinent role that radiology plays in the management of acute aortic diseases, with contrast-enhanced computed tomography angiography (CTA) being the most rapid and robust imaging technique.
Keywords: Acute aortic syndrome (AAS); aortic aneurysm; aortic dissection (AD); aortic emergencies; aortic rupture.
Conflict of interest statement
Conflicts of Interest: The authors have no conflicts of interest to declare.
Figures




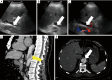
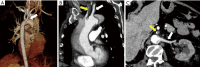
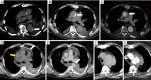
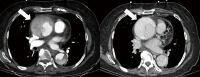


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
