miR-144-5p Enhances the Radiosensitivity of Non-Small-Cell Lung Cancer Cells via Targeting ATF2
- PMID: 29850528
- PMCID: PMC5925000
- DOI: 10.1155/2018/5109497
miR-144-5p Enhances the Radiosensitivity of Non-Small-Cell Lung Cancer Cells via Targeting ATF2
Abstract
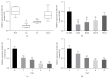
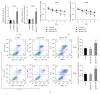
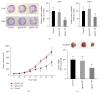
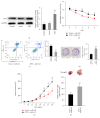
MicroRNAs (miRNAs or miRs) regulate gene expression at the posttranscriptional level and are involved in many biological processes such as cell proliferation and migration, stem cell differentiation, inflammation, and apoptosis. In particular, miR-144-3p is downregulated in various cancers, and its overexpression inhibits the proliferation and metastasis of cancer cells. However, the role of miR-144-5p in non-small-cell lung cancer (NSCLC), especially radiosensitivity, is unknown. In this study, we found that miR-144-5p was downregulated in NSCLC clinical specimens as well as NSCLC cell lines exposed to radiation. Enhanced expression of miR-144-5p promoted the radiosensitivity of NSCLC cells in vitro and A549 cell mouse xenografts in vivo. Furthermore, we identified activating transcription factor 2 (ATF2) as the direct and functional target of miR-144-5p using integrated bioinformatics analysis and a luciferase reporter assay. In addition, restoration of ATF2 expression inhibited miR-144-5p-induced NSCLC cell sensitivity to radiation in vitro and in vivo. Our findings suggest that deregulation of the miR-144-5p/ATF2 axis plays an important role in NSCLC cell radiosensitivity, thus representing a new potential therapeutic target for NSCLC.
Figures

Similar articles
-
6'-O-galloylpaeoniflorin regulates proliferation and metastasis of non-small cell lung cancer through AMPK/miR-299-5p/ATF2 axis.Respir Res. 2020 Feb 3;21(1):39. doi: 10.1186/s12931-020-1277-6. Respir Res. 2020. PMID: 32014006 Free PMC article.
-
Exosomal microRNA-26b-5p down-regulates ATF2 to enhance radiosensitivity of lung adenocarcinoma cells.J Cell Mol Med. 2020 Jul;24(14):7730-7742. doi: 10.1111/jcmm.15402. Epub 2020 May 31. J Cell Mol Med. 2020. PMID: 32476275 Free PMC article.
-
MiR-124 Inhibits Growth and Enhances Radiation-Induced Apoptosis in Non-Small Cell Lung Cancer by Inhibiting STAT3.Cell Physiol Biochem. 2017;44(5):2017-2028. doi: 10.1159/000485907. Epub 2017 Dec 11. Cell Physiol Biochem. 2017. PMID: 29237164
-
The activating transcription factor 2: an influencer of cancer progression.Mutagenesis. 2019 Dec 19;34(5-6):375-389. doi: 10.1093/mutage/gez041. Mutagenesis. 2019. PMID: 31799611 Free PMC article. Review.
-
MicroRNAs in the ionizing radiation response and in radiotherapy.Curr Opin Genet Dev. 2013 Feb;23(1):12-9. doi: 10.1016/j.gde.2013.01.002. Epub 2013 Feb 28. Curr Opin Genet Dev. 2013. PMID: 23453900 Free PMC article. Review.
Cited by
-
Tetramethylpyrazine Inhibits the Proliferation and Invasion of Glioma Cells by Regulating the UBL7-AS1/miR-144-3p Pathway.Evid Based Complement Alternat Med. 2022 Aug 22;2022:5261285. doi: 10.1155/2022/5261285. eCollection 2022. Evid Based Complement Alternat Med. 2022. Retraction in: Evid Based Complement Alternat Med. 2023 Dec 13;2023:9812814. doi: 10.1155/2023/9812814. PMID: 36045665 Free PMC article. Retracted.
-
[Plasma miRNA expression in patients with genetically confirmed multiple endocrine neoplasia type 1 syndrome and its phenocopies].Probl Endokrinol (Mosk). 2024 Jan 24;69(6):70-85. doi: 10.14341/probl13357. Probl Endokrinol (Mosk). 2024. PMID: 38311997 Free PMC article. Russian.
-
Effect of miR-144-5p on the proliferation, migration, invasion and apoptosis of human umbilical vein endothelial cells by targeting RICTOR and its related mechanisms.Exp Ther Med. 2020 Mar;19(3):1817-1823. doi: 10.3892/etm.2019.8369. Epub 2019 Dec 23. Exp Ther Med. 2020. PMID: 32104237 Free PMC article.
-
MiR-1246 Promotes Metastasis and Invasion of A549 cells by Targeting GSK-3β‒Mediated Wnt/β-Catenin Pathway.Cancer Res Treat. 2019 Oct;51(4):1420-1429. doi: 10.4143/crt.2018.638. Epub 2019 Mar 4. Cancer Res Treat. 2019. PMID: 30913872 Free PMC article.
-
Host-dependent alteration of the gut microbiota: the role of luminal microRNAs.Microbiome Res Rep. 2025 Feb 22;4(1):15. doi: 10.20517/mrr.2024.46. eCollection 2025. Microbiome Res Rep. 2025. PMID: 40207285 Free PMC article. Review.
References
-
- Lee Y. S., Oh J.-H., Yoon S., et al. Differential gene expression profiles of radioresistant non-small-cell lung cancer cell lines established by fractionated irradiation: tumor protein p53-inducible protein 3 confers sensitivity to ionizing radiation. International Journal of Radiation Oncology • Biology • Physics. 2010;77(3):858–866. doi: 10.1016/j.ijrobp.2009.12.076. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

