Pruritus: Progress toward Pathogenesis and Treatment
- PMID: 29850592
- PMCID: PMC5925168
- DOI: 10.1155/2018/9625936
Pruritus: Progress toward Pathogenesis and Treatment
Abstract
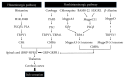
Pruritus, the most common cutaneous symptom, is widely seen in many skin complaints. It is an uncomfortable feeling on the skin and sometimes impairs patients' quality of life. At present, the specific mechanism of pruritus still remains unclear. Antihistamines, which are usually used to relieve pruritus, ineffectively work in some patients with itching. Recent evidence has suggested that, apart from histamine, many mediators and signaling pathways are involved in the pathogenesis of pruritus. Various therapeutic options for itching correspondingly have been developed. In this review, we summarize the updated pathogenesis and therapeutic strategies for pruritus.
Figures

References
-
- Olek-Hrab K., Hrab M., Szyfter-Harris J., Adamski Z. Pruritus in selected dermatoses. European Review for Medical and Pharmacological Sciences. 2016;20(17):3628–3641. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

