Reduction of myocardial ischaemia-reperfusion injury by inactivating oxidized phospholipids
- PMID: 29850765
- PMCID: PMC6302283
- DOI: 10.1093/cvr/cvy136
Reduction of myocardial ischaemia-reperfusion injury by inactivating oxidized phospholipids
Abstract
Aims: Myocardial ischaemia followed by reperfusion (IR) causes an oxidative burst resulting in cellular dysfunction. Little is known about the impact of oxidative stress on cardiomyocyte lipids and their role in cardiac cell death. Our goal was to identify oxidized phosphatidylcholine-containing phospholipids (OxPL) generated during IR, and to determine their impact on cell viability and myocardial infarct size.
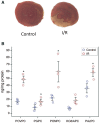
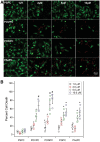
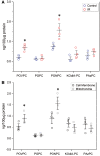
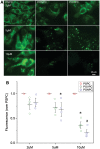
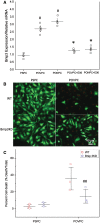
Methods and results: OxPL were quantitated in isolated rat cardiomyocytes using mass spectrophotometry following 24 h of IR. Cardiomyocyte cell death was quantitated following exogenously added OxPL and in the absence or presence of E06, a 'natural' murine monoclonal antibody that binds to the PC headgroup of OxPL. The impact of OxPL on mitochondria in cardiomyocytes was also determined using cell fractionation and Bnip expression. Transgenic Ldlr-/- mice, overexpressing a single-chain variable fragment of E06 (Ldlr-/--E06-scFv-Tg) were used to assess the effect of inactivating endogenously generated OxPL in vivo on myocardial infarct size. Following IR in vitro, isolated rat cardiomyocytes showed a significant increase in the specific OxPLs PONPC, POVPC, PAzPC, and PGPC (P < 0.05 to P < 0.001 for all). Exogenously added OxPLs resulted in significant death of rat cardiomyocytes, an effect inhibited by E06 (percent cell death with added POVPC was 22.6 ± 4.14% and with PONPC was 25.3 ± 3.4% compared to 8.0 ± 1.6% and 6.4 ± 1.0%, respectively, with the addition of E06, P < 0.05 for both). IR increased mitochondrial content of OxPL in rat cardiomyocytes and also increased expression of Bcl-2 death protein 3 (Bnip3), which was inhibited in presence of E06. Notably cardiomyocytes with Bnip3 knock-down were protected against cytotoxic effects of OxPL. In mice exposed to myocardial IR in vivo, compared to Ldlr-/- mice, Ldlr-/--E06-scFv-Tg mice had significantly smaller myocardial infarct size normalized to area at risk (72.4 ± 21.9% vs. 47.7 ± 17.6%, P = 0.023).
Conclusions: OxPL are generated within cardiomyocytes during IR and have detrimental effects on cardiomyocyte viability. Inactivation of OxPL in vivo results in a reduction of infarct size.
Figures



Similar articles
-
Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice.Nature. 2018 Jun;558(7709):301-306. doi: 10.1038/s41586-018-0198-8. Epub 2018 Jun 6. Nature. 2018. PMID: 29875409 Free PMC article.
-
Oxidized phosphatidylcholines trigger ferroptosis in cardiomyocytes during ischemia-reperfusion injury.Am J Physiol Heart Circ Physiol. 2021 Mar 1;320(3):H1170-H1184. doi: 10.1152/ajpheart.00237.2020. Epub 2021 Jan 29. Am J Physiol Heart Circ Physiol. 2021. PMID: 33513080
-
Shen-fu Injection Modulates HIF- 1α/BNIP3-Mediated Mitophagy to Alleviate Myocardial Ischemia-Reperfusion Injury.Cardiovasc Toxicol. 2025 Jun;25(6):898-914. doi: 10.1007/s12012-025-09993-3. Epub 2025 Apr 17. Cardiovasc Toxicol. 2025. PMID: 40246789
-
Progress in cardiac research: from rebooting cardiac regeneration to a complete cell atlas of the heart.Cardiovasc Res. 2021 Aug 29;117(10):2161-2174. doi: 10.1093/cvr/cvab200. Cardiovasc Res. 2021. PMID: 34114614 Free PMC article. Review.
-
CHARMM GUI Membrane Builder for oxidized phospholipid membrane modeling and simulation.Curr Opin Struct Biol. 2024 Jun;86:102813. doi: 10.1016/j.sbi.2024.102813. Epub 2024 Apr 9. Curr Opin Struct Biol. 2024. PMID: 38598982 Free PMC article. Review.
Cited by
-
Molecular Mechanisms of Ferroptosis and Relevance to Cardiovascular Disease.Cells. 2022 Sep 1;11(17):2726. doi: 10.3390/cells11172726. Cells. 2022. PMID: 36078133 Free PMC article. Review.
-
Metabolomic characterization of myocardial ischemia-reperfusion injury in ST-segment elevation myocardial infarction patients undergoing percutaneous coronary intervention.Sci Rep. 2019 Aug 13;9(1):11742. doi: 10.1038/s41598-019-48227-9. Sci Rep. 2019. PMID: 31409856 Free PMC article.
-
The Cardio- and Neuroprotective Effects of Corvitin and 2-Oxoglutarate in Rats with Pituitrin-Isoproterenol-Induced Myocardial Damage.Biochem Res Int. 2018 Sep 3;2018:9302414. doi: 10.1155/2018/9302414. eCollection 2018. Biochem Res Int. 2018. PMID: 30254764 Free PMC article.
-
Exosomes Mediate Hippocampal and Cortical Neuronal Injury Induced by Hepatic Ischemia-Reperfusion Injury through Activating Pyroptosis in Rats.Oxid Med Cell Longev. 2019 Nov 13;2019:3753485. doi: 10.1155/2019/3753485. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31814872 Free PMC article.
-
Ferroptosis in heart failure.J Mol Cell Cardiol. 2022 Dec;173:141-153. doi: 10.1016/j.yjmcc.2022.10.004. Epub 2022 Oct 20. J Mol Cell Cardiol. 2022. PMID: 36273661 Free PMC article. Review.
References
-
- O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX.. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;127:e362–e425. - PubMed
-
- Cung TT, Morel O, Cayla G, Rioufol G, Garcia-Dorado D, Angoulvant D, Bonnefoy-Cudraz E, Guerin P, Elbaz M, Delarche N, Coste P, Vanzetto G, Metge M, Aupetit JF, Jouve B, Motreff P, Tron C, Labeque JN, Steg PG, Cottin Y, Range G, Clerc J, Claeys MJ, Coussement P, Prunier F, Moulin F, Roth O, Belle L, Dubois P, Barragan P, Gilard M, Piot C, Colin P, De Poli F, Morice MC, Ider O, Dubois-Rande JL, Unterseeh T, Le Breton H, Beard T, Blanchard D, Grollier G, Malquarti V, Staat P, Sudre A, Elmer E, Hansson MJ, Bergerot C, Boussaha I, Jossan C, Derumeaux G, Mewton N, Ovize M.. Cyclosporine before PCI in patients with acute myocardial infarction. N Engl J Med 2015;373:1021–1031. - PubMed
-
- Yellon DM, Hausenloy DJ.. Myocardial reperfusion injury. N Engl J Med 2007;357:1121–1135. - PubMed
-
- Kloner RA, Hale SL, Dai W, Shi J.. Cardioprotection: where to from here? Cardiovasc Drugs Ther 2017;31:53–61. - PubMed
-
- Gibson CM, Giugliano RP, Kloner RA, Bode C, Tendera M, Janosi A, Merkely B, Godlewski J, Halaby R, Korjian S, Daaboul Y, Chakrabarti AK, Spielman K, Neal BJ, Weaver WD.. EMBRACE STEMI study: a phase 2a trial to evaluate the safety, tolerability, and efficacy of intravenous MTP-131 on reperfusion injury in patients undergoing primary percutaneous coronary intervention. Eur Heart J 2016;37:1296–1303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

