Reduced risk of heart failure with intensified multifactorial intervention in individuals with type 2 diabetes and microalbuminuria: 21 years of follow-up in the randomised Steno-2 study
- PMID: 29850922
- PMCID: PMC6061176
- DOI: 10.1007/s00125-018-4642-y
Reduced risk of heart failure with intensified multifactorial intervention in individuals with type 2 diabetes and microalbuminuria: 21 years of follow-up in the randomised Steno-2 study
Abstract
Aims/hypothesis: In type 2 diabetes mellitus, heart failure is a frequent, potentially fatal and often forgotten complication. Glucose-lowering agents and adjuvant therapies modify the risk of heart failure. We recently reported that 7.8 years of intensified compared with conventional multifactorial intervention in individuals with type 2 diabetes and microalbuminuria in the Steno-2 study reduced the risk of cardiovascular disease and prolonged life over 21.2 years of follow-up. In this post hoc analysis, we examine the impact of intensified multifactorial intervention on the risk of hospitalisation for heart failure.
Methods: One hundred and sixty individuals were randomised to conventional or intensified multifactorial intervention, using sealed envelopes. The trial was conducted using the Prospective, Randomised, Open, Blinded Endpoints (PROBE) design. After 7.8 years, all individuals were offered intensified therapy and the study continued as an observational follow-up study for an additional 13.4 years. Heart-failure hospitalisations were adjudicated from patient records by an external expert committee blinded for treatment allocation. Event rates were compared using a Cox regression model adjusted for age and sex.
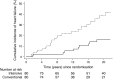
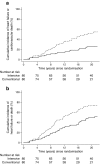
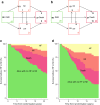
Results: Eighty patients were assigned to each treatment group. Ten patients undergoing intensive therapy vs 24 undergoing conventional therapy were hospitalised for heart failure during follow-up. The HR (95% CI) was 0.30 (0.14, 0.64), p = 0.002 in the intensive-therapy group compared with the conventional-therapy group. Including death in the endpoint did not lead to an alternate overall outcome; HR 0.51 (0.34, 0.76), p = 0.001. In a pooled cohort analysis, an increase in plasma N-terminal pro-B-type natriuretic peptide (NT-proBNP) during the first two years of the trial was associated with incident heart failure.
Conclusions/interpretation: Intensified, multifactorial intervention for 7.8 years in type 2 diabetic individuals with microalbuminuria reduced the risk of hospitalisation for heart failure by 70% during a total of 21.2 years of observation.
Trial registration: ClinicalTrials.gov NCT00320008.
Keywords: Complications; Heart failure; Microalbuminuria; Multifactorial intervention; NT-proBNP; Type 2 diabetes.
Conflict of interest statement
Since completion of the Steno-2 21.2 years follow-up data acquisition, data management and interpretation, JO has been employed by Novo Nordisk Scandinavia A/B, Region Denmark. PR reports having given lectures for Astra Zeneca, Bayer and Boehringer Ingelheim, has served as a consultant for AbbVie, Astra Zeneca, Bayer, Eli Lilly, Boehringer Ingelheim, Astellas, Janssen and Novo Nordisk (all fees given to the Steno Diabetes Center) and has equity interest in Novo Nordisk. HHP has equity interest in Merck and receives honoraria from AbbVie and Novartis. OP has equity interest in Novo Nordisk A/S. The Novo Nordisk Foundation Center for Basic Metabolic Research is an independent research centre at the University of Copenhagen partially funded by an unrestricted donation from the Novo Nordisk Foundation. PG, RR and LK declare that there is no duality of interest associated with this manuscript.
Figures
Similar articles
-
Intensified Multifactorial Intervention in Patients with Type 2 Diabetes Mellitus.Diabetes Metab J. 2023 Mar;47(2):185-197. doi: 10.4093/dmj.2022.0325. Epub 2023 Jan 12. Diabetes Metab J. 2023. PMID: 36631991 Free PMC article. Review.
-
Years of life gained by multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: 21 years follow-up on the Steno-2 randomised trial.Diabetologia. 2016 Nov;59(11):2298-2307. doi: 10.1007/s00125-016-4065-6. Epub 2016 Aug 16. Diabetologia. 2016. PMID: 27531506 Free PMC article. Clinical Trial.
-
Beneficial impact of intensified multifactorial intervention on risk of stroke: outcome of 21 years of follow-up in the randomised Steno-2 Study.Diabetologia. 2019 Sep;62(9):1575-1580. doi: 10.1007/s00125-019-4920-3. Epub 2019 Jun 1. Diabetologia. 2019. PMID: 31154479 Free PMC article. Clinical Trial.
-
A cost analysis of intensified vs conventional multifactorial therapy in individuals with type 2 diabetes: a post hoc analysis of the Steno-2 study.Diabetologia. 2019 Jan;62(1):147-155. doi: 10.1007/s00125-018-4739-3. Epub 2018 Oct 6. Diabetologia. 2019. PMID: 30293113 Free PMC article.
-
Effects of intensive interventions compared to standard care in people with type 2 diabetes and microalbuminuria on risk factors control and cardiovascular outcomes: A systematic review and meta-analysis of randomised controlled trials.Diabetes Res Clin Pract. 2018 Dec;146:76-84. doi: 10.1016/j.diabres.2018.10.002. Epub 2018 Oct 9. Diabetes Res Clin Pract. 2018. PMID: 30312714
Cited by
-
Diabetic vascular diseases: molecular mechanisms and therapeutic strategies.Signal Transduct Target Ther. 2023 Apr 10;8(1):152. doi: 10.1038/s41392-023-01400-z. Signal Transduct Target Ther. 2023. PMID: 37037849 Free PMC article. Review.
-
Intensified Multifactorial Intervention in Patients with Type 2 Diabetes Mellitus.Diabetes Metab J. 2023 Mar;47(2):185-197. doi: 10.4093/dmj.2022.0325. Epub 2023 Jan 12. Diabetes Metab J. 2023. PMID: 36631991 Free PMC article. Review.
-
Impact of Novel Guidelines on Multifactorial Control and Its Association with Mortality in Adult Patients with Hypertension and Newly Diagnosed Type 2 Diabetes: A 4-Year Prospective Multicenter Study.Int J Endocrinol. 2021 Sep 28;2021:9977840. doi: 10.1155/2021/9977840. eCollection 2021. Int J Endocrinol. 2021. PMID: 34621312 Free PMC article.
-
The Position of Gliclazide in the Evolving Landscapes and Disease Continuum of T2DM: A Collaborative Delphi Survey-Based Consensus from India.Diabetes Ther. 2021 Mar;12(3):679-695. doi: 10.1007/s13300-021-01002-4. Epub 2021 Jan 28. Diabetes Ther. 2021. PMID: 33511553 Free PMC article.
-
Age at diagnosis of type 2 diabetes and cardiovascular risk factor profile: A pooled analysis.World J Diabetes. 2022 Mar 15;13(3):260-271. doi: 10.4239/wjd.v13.i3.260. World J Diabetes. 2022. PMID: 35432761 Free PMC article.
References
-
- Johansson I, Edner M, Dahlstrom U, Nasman P, Ryden L, Norhammar A. Is the prognosis in patients with diabetes and heart failure a matter of unsatisfactory management? An observational study from the Swedish heart failure registry. Eur J Heart Fail. 2014;16:409–418. doi: 10.1002/ejhf.44. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

