Pharmacokinetics and pharmacodynamics of ticagrelor in subjects on hemodialysis and subjects with normal renal function
- PMID: 29850937
- PMCID: PMC6096709
- DOI: 10.1007/s00228-018-2484-7
Pharmacokinetics and pharmacodynamics of ticagrelor in subjects on hemodialysis and subjects with normal renal function
Abstract
Purpose: This single-dose, randomized, open-label, parallel-group, and crossover study assessed pharmacokinetics (PK), pharmacodynamics (PD), and safety of ticagrelor in subjects on hemodialysis versus healthy subjects.
Methods: Hemodialysis subjects were randomized, receiving a single ticagrelor 90-mg dose 1 day post-hemodialysis or just before hemodialysis, with an intervening washout of ≥ 7 days. Healthy subjects (creatinine clearance ≥ 90 mL/min) received a single ticagrelor 90-mg dose. PK, PD (P2Y12 reaction units [PRU], inhibition of platelet aggregation [IPA]), and safety were evaluated.
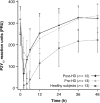
Results: Twenty-seven subjects (14 hemodialysis, 13 healthy) received ticagrelor. The mean maximum plasma concentration (Cmax) and area under the plasma concentration curve from time zero to infinity (AUC0-∞) of ticagrelor were 598.4 ng/mL and 3256.1 ng·h/mL, respectively, in pre-hemodialysis subjects; 560.3 ng/mL and 3015.1 ng·h/mL, respectively, in post-hemodialysis subjects; and 370.8 ng/mL and 2188.8 ng·h/mL, respectively, in healthy subjects. Cmax and AUC0-∞ of AR-C124910XX, the active metabolite, were 152.3 ng/mL and 1144.2 ng·h/mL, respectively, in pre-hemodialysis subjects; 130.8 ng/mL and 1127.8 ng·h/mL, respectively, in post-hemodialysis subjects; and 111.7 ng/mL and 1000.4 ng·h/mL, respectively, in healthy subjects. Mean IPA time curves over 24 h post-dose were almost indistinguishable for all three treatments. The greatest reduction in mean PRU occurred approximately 2 h post-dose for all three treatments. No safety or tolerability issues were identified.
Conclusion: Hemodialysis resulted in modestly higher exposure to ticagrelor and AR-C124910XX, with no clinically significant effect on PD or tolerability. Accordingly, no dose adjustment is required for hemodialysis patients. Timing of hemodialysis has little impact on ticagrelor PK, or the effect of ticagrelor on IPA.
Keywords: Hemodialysis; Pharmacodynamics; Pharmacokinetics; Ticagrelor.
Conflict of interest statement
Conflict of interest
S. Muldowney and N.D. Khan are employees and shareholders of AstraZeneca. Y. Zhao is a former consultant to AstraZeneca. R. Teng and J. Lu are former employees of AstraZeneca. J. Berg is an employee of DaVita Clinical Research and a principal investigator in the trial.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Figures



References
-
- O’Gara PT, Kushner FG, Ascheim DD et al (2013) 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 61:e78–e140 - PubMed
-
- Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S, ESC Scientific Document Group 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC) Eur Heart J. 2016;37:267–315. doi: 10.1093/eurheartj/ehv320. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

