Association of Genetic Variants With Response to Anti-Vascular Endothelial Growth Factor Therapy in Age-Related Macular Degeneration
- PMID: 29852030
- PMCID: PMC6142943
- DOI: 10.1001/jamaophthalmol.2018.2019
Association of Genetic Variants With Response to Anti-Vascular Endothelial Growth Factor Therapy in Age-Related Macular Degeneration
Abstract
Importance: Visual acuity (VA) outcomes differ considerably among patients with neovascular age-related macular degeneration (nAMD) treated with anti-vascular endothelial growth factor (VEGF) drugs. Identification of pharmacogenetic associations may help clinicians understand the mechanisms underlying this variability as well as pave the way for personalized treatment in nAMD.
Objective: To identify genetic factors associated with variability in the response to anti-VEGF therapy for patients with nAMD.
Design, setting, and participants: In this multicenter genome-wide association study, 678 patients with nAMD with genome-wide genotyping data were included in the discovery phase; 1380 additional patients with nAMD were genotyped for selected common variants in the replication phase. All participants received 3 monthly injections of bevacizumab or ranibizumab. Clinical data were evaluated for inclusion/exclusion criteria from October 2014 to October 2015, followed by data analysis from October 2015 to February 2016. For replication cohort genotyping, clinical data collection and analysis (including meta-analysis) was performed from March 2016 to April 2017.
Main outcomes and measures: Change in VA after the loading dose of 3 monthly anti-VEGF injections compared with baseline.
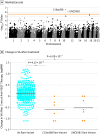
Results: Of the 2058 included patients, 1210 (58.8%) were women, and the mean (SD) age across all cohorts was 78 (7.4) years. Patients included in the discovery cohort and most of the patients in the replication cohorts were of European descent. The mean (SD) baseline VA was 51.3 (20.3) Early Treatment Diabetic Retinopathy Study (ETDRS) score letters, and the mean (SD) change in VA after the loading dose of 3 monthly injections was a gain of 5.1 (13.9) ETDRS score letters (ie, 1-line gain). Genome-wide single-variant analyses of common variants revealed 5 independent loci that reached a P value less than 10 × 10-5. After replication and meta-analysis of the lead variants, rs12138564 located in the CCT3 gene remained nominally associated with a better treatment outcome (ETDRS letter gain, 1.7; β, 0.034; SE, 0.008; P = 1.38 × 10-5). Genome-wide gene-based optimal unified sequence kernel association test of rare variants showed genome-wide significant associations for the C10orf88 (P = 4.22 × 10-7) and UNC93B1 (P = 6.09 × 10-7) genes, in both cases leading to a worse treatment outcome. Patients carrying rare variants in the C10orf88 and UNC93B1 genes lost a mean (SD) VA of 30.6 (17.4) ETDRS score letters (ie, loss of 6.09 lines) and 26.5 (13.8) ETDRS score letters (ie, loss of 5.29 lines), respectively, after 3 months of anti-VEGF treatment.
Conclusions and relevance: We propose that there is a limited contribution of common genetic variants to variability in nAMD treatment response. Our results suggest that rare protein-altering variants in the C10orf88 and UNC93B1 genes are associated with a worse response to anti-VEGF therapy in patients with nAMD, but these results require further validation in other cohorts.
Conflict of interest statement
Figures

Comment in
-
Understanding Variation in Response to Anti-Vascular Endothelial Growth Factor Therapy for Neovascular Age-Related Macular Degeneration.JAMA Ophthalmol. 2018 Aug 1;136(8):884-885. doi: 10.1001/jamaophthalmol.2018.0032. JAMA Ophthalmol. 2018. PMID: 29852031 No abstract available.
References
-
- Friedman DS, O’Colmain BJ, Muñoz B, et al. ; Eye Diseases Prevalence Research Group . Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564-572. - PubMed
-
- Ferris FL III, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984;102(11):1640-1642. - PubMed
-
- Rosenfeld PJ, Brown DM, Heier JS, et al. ; MARINA Study Group . Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419-1431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

