Stereotactic Body Radiation Therapy for Operable Early-Stage Lung Cancer: Findings From the NRG Oncology RTOG 0618 Trial
- PMID: 29852037
- PMCID: PMC6117102
- DOI: 10.1001/jamaoncol.2018.1251
Stereotactic Body Radiation Therapy for Operable Early-Stage Lung Cancer: Findings From the NRG Oncology RTOG 0618 Trial
Abstract
Importance: Stereotactic body radiation therapy (SBRT) has become a standard treatment for patients with medically inoperable early-stage lung cancer. However, its effectiveness in patients medically suitable for surgery is unclear.
Objective: To evaluate whether noninvasive SBRT delivered on an outpatient basis can safely eradicate lung cancer and cure selected patients with operable lung cancer, obviating the need for surgical resection.
Design, setting, and participants: Single-arm phase 2 NRG Oncology Radiation Therapy Oncology Group 0618 study enrolled patients from December 2007 to May 2010 with median follow-up of 48.1 months (range, 15.4-73.7 months). The setting was a multicenter North American academic and community practice cancer center consortium. Patients had operable biopsy-proven peripheral T1 to T2, N0, M0 non-small cell tumors no more than 5 cm in diameter, forced expiratory volume in 1 second (FEV1) and diffusing capacity greater than 35% predicted, arterial oxygen tension greater than 60 mm Hg, arterial carbon dioxide tension less than 50 mm Hg, and no severe medical problems. The data analysis was performed in October 2014.
Interventions: The SBRT prescription dose was 54 Gy delivered in 3 18-Gy fractions over 1.5 to 2.0 weeks.
Main outcomes and measures: Primary end point was primary tumor control, with survival, adverse events, and the incidence and outcome of surgical salvage as secondary end points.
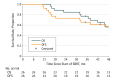
Results: Of 33 patients accrued, 26 were evaluable (23 T1 and 3 T2 tumors; 15 [58%] male; median age, 72.5 [range, 54-88] years). Median FEV1 and diffusing capacity of the lung for carbon monoxide at enrollment were 72.5% (range, 38%-136%) and 68% (range, 22%-96%) of predicted, respectively. Only 1 patient had a primary tumor recurrence. Involved lobe failure, the other component defining local failure, did not occur in any patient, so the estimated 4-year primary tumor control and local control rate were both 96% (95% CI, 83%-100%). As per protocol guidelines, the single patient with local recurrence underwent salvage lobectomy 1.2 years after SBRT, complicated by a grade 4 cardiac arrhythmia. The 4-year estimates of disease-free and overall survival were 57% (95% CI, 36%-74%) and 56% (95% CI, 35%-73%), respectively. Median overall survival was 55.2 months (95% CI, 37.7 months to not reached). Protocol-specified treatment-related grade 3, 4, and 5 adverse events were reported in 2 (8%; 95% CI, 0.1%-25%), 0, and 0 patients, respectively.
Conclusions and relevance: As given, SBRT appears to be associated with a high rate of primary tumor control, low treatment-related morbidity, and infrequent need for surgical salvage in patients with operable early-stage lung cancer.
Trial registration: ClinicalTrials.gov Identifier: NCT00551369.
Conflict of interest statement
Figures

References
-
- Timmerman RD, Kavanagh BD, Cho LC, Papiez L, Xing L. Stereotactic body radiation therapy in multiple organ sites. J Clin Oncol. 2007;25(8):947-952. - PubMed
-
- Potters L, Steinberg M, Rose C, et al. ; American Society for Therapeutic Radiology and Oncology; American College of Radiology . American Society for Therapeutic Radiology and Oncology and American College of Radiology practice guideline for the performance of stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2004;60(4):1026-1032. - PubMed
-
- Huang L, Park K, Boike T, et al. A study on the dosimetric accuracy of treatment planning for stereotactic body radiation therapy of lung cancer using average and maximum intensity projection images. Radiother Oncol. 2010;96(1):48-54. - PubMed
-
- Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol. 2009;27(20):3290-3296. - PubMed
-
- Nagata Y, Hiraoka M, Shibata T, et al. Stereotactic body radiation therapy for T1N0M0 non-small cell lung cancer: first report for inoperable population of a phase II trial by Japan Clinical Oncology Group (JCOG 0403). Int J Radiat Oncol Biol Phys. 2012;84(3 suppl):S46.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

