Nicotine replacement therapy versus control for smoking cessation
- PMID: 29852054
- PMCID: PMC6353172
- DOI: 10.1002/14651858.CD000146.pub5
Nicotine replacement therapy versus control for smoking cessation
Abstract
Background: Nicotine replacement therapy (NRT) aims to temporarily replace much of the nicotine from cigarettes to reduce motivation to smoke and nicotine withdrawal symptoms, thus easing the transition from cigarette smoking to complete abstinence.
Objectives: To determine the effectiveness and safety of nicotine replacement therapy (NRT), including gum, transdermal patch, intranasal spray and inhaled and oral preparations, for achieving long-term smoking cessation, compared to placebo or 'no NRT' interventions.
Search methods: We searched the Cochrane Tobacco Addiction Group trials register for papers mentioning 'NRT' or any type of nicotine replacement therapy in the title, abstract or keywords. Date of most recent search is July 2017.
Selection criteria: Randomized trials in people motivated to quit which compared NRT to placebo or to no treatment. We excluded trials that did not report cessation rates, and those with follow-up of less than six months, except for those in pregnancy (where less than six months, these were excluded from the main analysis). We recorded adverse events from included and excluded studies that compared NRT with placebo. Studies comparing different types, durations, and doses of NRT, and studies comparing NRT to other pharmacotherapies, are covered in separate reviews.
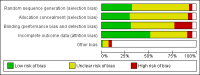
Data collection and analysis: Screening, data extraction and 'Risk of bias' assessment followed standard Cochrane methods. The main outcome measure was abstinence from smoking after at least six months of follow-up. We used the most rigorous definition of abstinence for each trial, and biochemically validated rates if available. We calculated the risk ratio (RR) for each study. Where appropriate, we performed meta-analysis using a Mantel-Haenszel fixed-effect model.
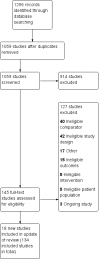
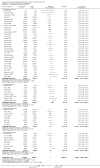
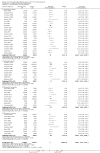
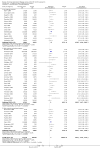
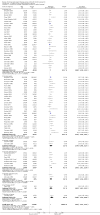
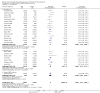
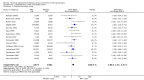
Main results: We identified 136 studies; 133 with 64,640 participants contributed to the primary comparison between any type of NRT and a placebo or non-NRT control group. The majority of studies were conducted in adults and had similar numbers of men and women. People enrolled in the studies typically smoked at least 15 cigarettes a day at the start of the studies. We judged the evidence to be of high quality; we judged most studies to be at high or unclear risk of bias but restricting the analysis to only those studies at low risk of bias did not significantly alter the result. The RR of abstinence for any form of NRT relative to control was 1.55 (95% confidence interval (CI) 1.49 to 1.61). The pooled RRs for each type were 1.49 (95% CI 1.40 to 1.60, 56 trials, 22,581 participants) for nicotine gum; 1.64 (95% CI 1.53 to 1.75, 51 trials, 25,754 participants) for nicotine patch; 1.52 (95% CI 1.32 to 1.74, 8 trials, 4439 participants) for oral tablets/lozenges; 1.90 (95% CI 1.36 to 2.67, 4 trials, 976 participants) for nicotine inhalator; and 2.02 (95% CI 1.49 to 2.73, 4 trials, 887 participants) for nicotine nasal spray. The effects were largely independent of the definition of abstinence, the intensity of additional support provided or the setting in which the NRT was offered. A subset of six trials conducted in pregnant women found a statistically significant benefit of NRT on abstinence close to the time of delivery (RR 1.32, 95% CI 1.04 to 1.69; 2129 participants); in the four trials that followed up participants post-partum the result was no longer statistically significant (RR 1.29, 95% CI 0.90 to 1.86; 1675 participants). Adverse events from using NRT were related to the type of product, and include skin irritation from patches and irritation to the inside of the mouth from gum and tablets. Attempts to quantitatively synthesize the incidence of various adverse effects were hindered by extensive variation in reporting the nature, timing and duration of symptoms. The odds ratio (OR) of chest pains or palpitations for any form of NRT relative to control was 1.88 (95% CI 1.37 to 2.57, 15 included and excluded trials, 11,074 participants). However, chest pains and palpitations were rare in both groups and serious adverse events were extremely rare.
Authors' conclusions: There is high-quality evidence that all of the licensed forms of NRT (gum, transdermal patch, nasal spray, inhalator and sublingual tablets/lozenges) can help people who make a quit attempt to increase their chances of successfully stopping smoking. NRTs increase the rate of quitting by 50% to 60%, regardless of setting, and further research is very unlikely to change our confidence in the estimate of the effect. The relative effectiveness of NRT appears to be largely independent of the intensity of additional support provided to the individual. Provision of more intense levels of support, although beneficial in facilitating the likelihood of quitting, is not essential to the success of NRT. NRT often causes minor irritation of the site through which it is administered, and in rare cases can cause non-ischaemic chest pain and palpitations.
Conflict of interest statement
CB was involved in a trial on pre‐cessation use of NRT (Bullen 2010)
SCC none known
JHB none known
TL none known
WY none known
Figures








Update of
-
Nicotine replacement therapy for smoking cessation.Cochrane Database Syst Rev. 2012 Nov 14;11:CD000146. doi: 10.1002/14651858.CD000146.pub4. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2018 May 31;5:CD000146. doi: 10.1002/14651858.CD000146.pub5. PMID: 23152200 Updated.
References
References to studies included in this review
Abelin 1989 {published data only}
-
- Abelin T, Buehler A, Muller P, Vesanen K, Imhof PR. Controlled trial of transdermal nicotine patch in tobacco withdrawal. Lancet 1989;1(8628):7‐10. - PubMed
-
- Abelin T, Ehrsam R, Buhler‐Reichert A, Imhof PR, Muller P, Thommen A. Effectiveness of a transdermal nicotine system in smoking cessation studies. Methods and Findings in Experimental and Clinical Pharmacology 1989;11(3):205‐14. - PubMed
-
- Muller P, Abelin T, Ehrsam R, Imhof P, Howald H, Mauli D. The use of transdermal nicotine in smoking cessation. Lung 1990;168 Suppl:445‐53. - PubMed
Ahluwalia 1998 {published data only}
Ahluwalia 2006 {published data only}
-
- Ahluwalia JS, Okuyemi K, Nollen N, Choi WS, Kaur H, Pulvers K, et al. The effects of nicotine gum and counseling among African American light smokers: a 2 x 2 factorial design. Addiction 2006;101(6):883‐91. - PubMed
-
- Okuyemi KS, Cox LS, Nollen NL, Snow TM, Kaur H, Choi W, et al. Baseline characteristics and recruitment strategies in a randomized clinical trial of African‐American light smokers. American Journal of Health Promotion 2007;21(3):183‐91. - PubMed
-
- Okuyemi KS, Faseru B, Sanderson Cox L, Bronars CA, Ahluwalia JS. Relationship between menthol cigarettes and smoking cessation among African American light smokers. Addiction 2007;102(12):1979‐86. - PubMed
Anthenelli 2016 {published and unpublished data}
-
- Anthenelli R, Benowitz N, West R, Aubin L, McRae T, Lawrence D, et al. Reports of suicidal ideation and behavior in the EAGLES trial. Society for Research on Nicotine and Tobacco; 2016 March 03‐05; USA. 2016; Vol. SYM5D:8.
-
- Anthenelli RM, Benowitz NL, West R, Aubin L, McRae T, Lawrence D, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double‐blind, randomised, placebo‐controlled clinical trial. Lancet 2016; Vol. 387, issue 10037:2507‐20. [DOI: ] - PubMed
-
- Benowitz N, Evins AE, West R, Aubin L, McRae T, Lawrence D, et al. EAGLES trial: study design and neuropsychiatric safety results. Society for Research on Nicotine and Tobacco; 2016 March 03‐05; USA. 2016; Vol. SYM5B:7.
-
- NCT01456936. A phase 4, randomized, double blind, active and placebo controlled multicenter study evaluating the neuropsychiatric safety and efficacy of 12 weeks varenicline tartrate 1mg BID and bupropion hydrochloride 150 mg BID for smoking cessation in subjects with and without a history of psychiatric disorders [EAGLES]. clinicaltrials.gov/ct2/show/NCT01456936 (first received 21 October 2011).
-
- Prochaska J. Neuropsychiatric risk concerns in the context of smoking, quitting, and cessation pharmacotherapy use. Society for Research on Nicotine and Tobacco; 2016 March 03‐05; USA. 2016; Vol. SYM5A:7.
Areechon 1988 {published data only}
-
- Areechon W, Punnotok J. Smoking cessation through the use of nicotine chewing gum: a double‐blind trial in Thailand. Clinical Therapeutics 1988;10:183‐6. - PubMed
Berlin 2014 {published data only}
-
- Lancaster T. In pregnant smokers, the nicotine patch did not increase abstinence or birthweight more than placebo. Annals of Internal Medicine 2014;160(12):JC11. - PubMed
Blondal 1989 {published data only}
-
- Blondal T. Controlled trial of nicotine polacrilex gum with supportive measures. Archives of Internal Medicine 1989;149:1818‐21. - PubMed
Blondal 1997 {published data only}
-
- Blondal T, Franzon M, Westin A. A double‐blind randomized trial of nicotine nasal spray as an aid in smoking cessation. European Respiratory Journal 1997;10:1585‐90. - PubMed
-
- Blondal T, Olafsdottir I, Gunnarsdottir R, Franzon M, Westin A. Controlled trial of nicotine nasal spray as an aid to stopping smoking [abstract]. European Respiratory Journal 1993;6(Suppl 17):631S.
Bolliger 2000b {published data only}
-
- Bolliger CT. Practical experiences in smoking reduction and cessation. Addiction 2000;95(1 S1):S19‐S24. - PubMed
-
- Bolliger CT, Zellweger JP, Danielsson T, Biljon X, Robidou A, Westin A, et al. Influence of long‐term smoking reduction on health risk markers and quality of life. Nicotine & Tobacco Research 2002;4:433‐9. - PubMed
Brantmark 1973b {published data only}
-
- Brantmark B, Ohlin P, Westling H. Nicotine‐containing chewing gum as an anti‐smoking aid. Psychopharmacologia 1973;31:191‐200. - PubMed
Br Thor Society 1983 {published data only}
-
- Research Committee of the British Thoracic Society. Comparison of four methods of smoking withdrawal in patients with smoking related diseases. Report by a subcommittee of the Research Committee of the British Thoracic Society. British Medical Journal (Clinical research ed.) 1983;286(6365):595‐7. - PMC - PubMed
Buchkremer 1988 {published data only}
-
- Buchkremer G, Bents H, Horstmann M, Opitz K, Tolle R. Combination of behavioral smoking cessation with transdermal nicotine substitution. Addictive Behaviors 1989;14:229‐38. - PubMed
-
- Buchkremer G, Bents H, Minneker E, Opitz K. Long‐term effects of a combination of transdermal nicotine administration with behavior therapy for smoking cessation [Langfristige Effekte einer Kombination von transdermaler Nikotinzufuhr mit Verhaltenstherapie zur Raucherentwohnung]. Nervenarzt 1988;59:488‐90. - PubMed
Bullen 2010 {published data only}
-
- Bullen C, Howe C, Lin RB, Grigg M, Laugesen M, McRobbie H, et al. Pre‐cessation nicotine replacement therapy: pragmatic randomized trial. Addiction 2010;105(8):1474‐83. - PubMed
Campbell 1987 {published data only}
-
- Campbell IA, Lyons E, Prescott R. Do nicotine chewing‐gum and postal encouragement add to doctors' advice. Practitioner 1987;231:114‐7. - PubMed
Campbell 1991 {published data only}
-
- Campbell IA, Prescott RJ, Tjeder‐Burton SM. Smoking cessation in hospital patients given repeated advice plus nicotine or placebo chewing gum. Respiratory Medicine 1991;85:155‐7. - PubMed
Campbell 1996 {published data only}
-
- Burton S, Campbell IA, Prescott RJ. Nicotine patches versus placebo in 235 hospital patients [abstract 191]. 8th World Conference on Tobacco or Health; 1992 Mar 30‐Apr 03; Buenos Aires, Argentina. 1992.
-
- Campbell IA, Prescott RJ, Tjeder‐Burton SM. Transdermal nicotine plus support in patients attending hospital with smoking‐related diseases: a placebo‐controlled study. Respiratory Medicine 1996;90:47‐51. - PubMed
CEASE 1999 {published data only}
-
- Tønnesen P, Paoletti P, Gustavsson G, Russell MA, Saracci R, Gulsvik A, et al. Higher dosage nicotine patches increase one‐year smoking cessation rates: Results from the European CEASE trial. European Respiratory Journal 1999;13:238‐46. - PubMed
Cinciripini 1996 {published data only}
-
- Cinciripini PM, Cinciripini LG, Wallfisch A, Haque W. Behavior therapy and the transdermal nicotine patch: Effects on cessation outcome, affect, and coping. Journal of Consulting and Clinical Psychology 1996;64:314‐23. - PubMed
-
- Cinciripini PM, Wetter DW, Fouladi RT, Blalock JA, Carter BL, Cinciripini LG, et al. The effects of depressed mood on smoking cessation: mediation by postcessation self‐efficacy. Journal of Consulting and Clinical Psychology 2003;71:292‐301. - PubMed
Clavel 1985 {published data only}
-
- Clavel F, Benhamou S. Tobacco withdrawal. Comparison of the efficacy of various methods. Intermediate results of a comparative study [Désintoxcation tabagique. Comparison de l'efficacité de differentes méthodes. Résultats intermédiaires d'une étude comparative]. Presse Médicale 1984;13:975‐7. - PubMed
Clavel‐Chapelon 1992 {published data only}
-
- Clavel‐Chapelon F, Paoletti C, Benhamou S. A randomised 2 x 2 factorial design to evaluate different smoking cessation methods. Revue d'Epidemiologie et de Sante Publique 1992;40:187‐90. - PubMed
Coleman 2012 {published data only}
-
- Coleman T, Cooper S, Thornton JG, Grainge MJ, Watts K Britton J, et al. A randomized trial of nicotine‐replacement therapy patches in pregnancy. The New England Journal of Medicine 2012;366(9):808‐19. - PubMed
-
- Coleman T, Cooper S, Thornton JG, Grainge MJ, Watts K, Britton J, et al. A randomized trial of nicotine‐replacement therapy patches in pregnancy. Obstetrical & Gynecological Survey 2012;67(7):387‐8.
-
- Cooper S, Lewis S, Thornton JG, Marlow N, Watts K, Britton J, et al. The SNAP trial: a randomised placebo‐controlled trial of nicotine replacement therapy in pregnancy‐‐clinical effectiveness and safety until 2 years after delivery, with economic evaluation. Health Technology Assessment (Winchester, England) 2014;18(54):1‐128. - PMC - PubMed
Cooper 2005 {published data only}
-
- Cooper TV, Klesges RC, Debon MW, Zbikowski SM, Johnson KC, Clemens LH. A placebo controlled randomized trial of the effects of phenylpropanolamine and nicotine gum on cessation rates and postcessation weight gain in women. Addictive Behaviors 2005;30:61‐75. - PubMed
-
- Cooper TV, Montgomery GV, Debon MW, Zbikowski SM, Klesges RC, Johnson KC. The effects of PPA and nicotine gum on cessation rates and post cessation weight gain in women [POS3‐46]. Society for Research on Nicotine and Tobacco 9th Annual Meeting; 2003; New Orleans, LA. 2003.
Cummins 2016 {published data only}
Cunningham 2016 {published data only}
-
- Cunningham JA, Kushnir V, Selby P, Tyndale RF, Zawertailo L, Leatherdale ST. Effect of mailing nicotine patches on tobacco cessation among adult smokers: a randomized clinical trial. JAMA Internal Medicine 2016;176(2):184‐90. - PubMed
-
- Kushnir V, Sproule BA, Cunningham JA. Mailed distribution of free nicotine patches without behavioral support: Predictors of use and cessation. Addictive Behaviors 2017;67:73‐8. - PubMed
-
- Kushnir V, Sproule BA, Zawertailo L, Selby P, Tyndale RF, Leatherdale ST, et al. Impact of self‐reported lifetime depression or anxiety on effectiveness of mass distribution of nicotine patches. Tobacco Control 2016;26(5):526‐33. - PubMed
-
- Wise J. Mailing free nicotine patches to smokers reduces smoking rates, study shows. BMJ (Clinical research ed.) 2016;352:i468. - PubMed
Daughton 1991 {published data only}
-
- Daughton DM, Heatley SA, Prendergast JJ, Causey D, Knowles M, Rolf CN, et al. Effect of transdermal nicotine delivery as an adjunct to low‐intervention smoking cessation therapy. A randomized, placebo‐controlled, double‐blind study. Archives of Internal Medicine 1991;151:749‐52. - PubMed
-
- Daughton DM, Heatley SA, Prendergast JJ, Causey D, Knowles M, Rolf CN, et al. Effects of transdermal nicotine as an adjunct in smoking cessation therapy. A double‐blind randomized study controlled with placebo [Effetti del rilascio transdermico di nicotina come terapia di supporto per lo svezzamento dal fumo di sigaretta. Uno studio randomizzato in doppio cieco con controlli trattati con placebo.]. Archivio Monaldi 1992;47:17‐29. - PubMed
Daughton 1998 {published data only}
-
- Daughton D, Susman J, Sitorius M, Belenky S, Millatmal T, Nowak R, et al. Transdermal nicotine therapy and primary care. Importance of counseling, demographic, and participant selection factors on 1‐year quit rates. The Nebraska Primary Practice Smoking Cessation Trial Group. Archives of Family Medicine 1998;7:425‐30. - PubMed
Dautzenberg 2001 {published and unpublished data}
-
- Dautzenberg B, Peiffer G, Toulouse F, Yvinec MJ, Jacob N, Kienzler JL. Randomized trial assessment of nicotinell lozenge 1mg, a new oral nicotine replacement therapy. Society for Research on Nicotine and Tobacco 3rd European Conference; 2001; Paris, France. 2001:55.
-
- Dautzenberg B, Ruff F, Vaucher M, Maillon P, Jacob N, Kienzler JL, et al. First demonstration of the good efficacy/safety ratio of Nicotinell® 1mg Lozenge (NL 1mg), a new form of nicotine substitution, by randomised clinical trial. European Respiratory Journal 2001;18 Suppl 33:12s.
Davidson 1998 {published data only}
-
- Davidson M, Epstein M, Burt R, Schaefer C, Whitworth G, McDonald A. Efficacy and safety of an over‐the‐counter transdermal nicotine patch as an aid for smoking cessation. Archives of Family Medicine 1998;7:569‐74. - PubMed
Ehrsam 1991 {published data only}
-
- Abelin T, Ehrsam R, Buhler‐Reichert A, Imhof PR, Muller P, Thommen A, et al. Effectiveness of a transdermal nicotine system in smoking cessation studies. Methods and Findings in Experimental and Clinical Pharmacology 1989;11:205‐14. - PubMed
-
- Abelin T, Ehrsam R, Imhof P, Muller P, Howald H. Clinical experience with a transdermal nicotine system in healthy nicotine‐dependent smokers. In: Wilhemsen L editor(s). Smoking as a Cardiovascular Risk Factor ‐ New Strategies for Smoking Cessation. Hogrefe & Huber, 1991:35‐46.
-
- Ehrsam RE, Buhler A, Muller P, Mauli D, Schumacher PM, Howald H, et al. Weaning of young smokers using a transdermal nicotine patch [Entwohnung junger Raucher mit Hilfe eines transdermalen Nikotinpflasters]. Schweizerische Rundschau fur Medizin Praxis 1991;80:145‐50. - PubMed
-
- Muller P, Abelin T, Ehrsam R, Imhof P, Howald H, Mauli D. The use of transdermal nicotine in smoking cessation. Lung 1990;168:445‐53. - PubMed
El‐Mohandes 2013 {published data only}
Fagerström 1982 {published data only}
-
- Fagerström KO. A comparison of psychological and pharmacological treatment in smoking cessation. Journal of Behavioral Medicine 1982;5:343‐51. - PubMed
-
- Fagerström KO. Tolerance, withdrawal and dependence on tobacco and smoking termination. International Review of Applied Psychology 1983;32:29‐52.
Fagerström 1984 {published data only}
-
- Fagerström KO. Effects of nicotine chewing gum and follow‐up appointments in physician‐based smoking cessation. Preventive Medicine 1984;13:517‐27. - PubMed
Fee 1982 {published data only}
-
- Fee WM, Stewart MJ. A controlled trial of nicotine chewing gum in a smoking withdrawal clinic. Practitioner 1982;226:148‐51. - PubMed
Fiore 1994a {published data only}
-
- Elan Pharmaceutical Research Corp. NDA 19‐983 for Approval of PROSTEP. Study 90‐03 1992.
-
- Fiore MC, Kenford SL, Jorenby DE, Wetter DW, Smith SS, Baker TB. Two studies of the clinical effectiveness of the nicotine patch with different counseling treatments. Chest 1994;105:524‐33. - PubMed
-
- Kenford SL, Fiore MC, Jorenby DE, Smith SS, Wetter D, Baker TB. Predicting smoking cessation. Who will quit with and without the nicotine patch. JAMA 1994;271:589‐94. - PubMed
Fiore 1994b {published data only}
-
- Fiore MC, Kenford SL, Jorenby DE, Wetter DW, Smith SS, Baker TB. Two studies of the clinical effectiveness of the nicotine patch with different counseling treatments. Chest 1994;105:524‐33. - PubMed
Fortmann 1995 {published data only}
-
- Fortmann SP, Killen JD. Nicotine gum and self‐help behavioral treatment for smoking relapse prevention ‐ resuIts from a trial using population‐based recruitment. Journal of Consulting and Clinical Psychology 1995;63:460‐8. - PubMed
Fraser 2014 {published data only}
Gallagher 2007 {published data only}
-
- Gallagher SM, Penn PE, Schindler E. Smoking cessation in persons with schizophrenia and other serious mental illness (PA1‐5). Society for Research on Nicotine and Tobacco 12th Annual Meeting; 2006; Orlando, Florida, USA. 2006.
-
- Gallagher SM, Penn PE, Schindler E, Layne W. A comparison of smoking cessation treatments for persons with schizophrenia and other serious mental illnesses. Journal of Psychoactive Drugs 2007;39(4):487‐97. - PubMed
García 1989 {published data only}
-
- Quílez García C, Hernando Arizaleta L, Rubio Díaz A, Estruch Riba J, Fornés Ramis MV. Treatment for smoking with nicotine gum in primary care. A double blind trial [Tratamiento del tabaquismo, con chicle de nicotina, en atencion primaria. Estudio a doble ciego]. Revista Clinica Espanola 1993;192(4):157‐61. - PubMed
-
- Quílez García C, Hernando Arizaleta L, Rubio Díaz A, Granero Fernández EJ, Vila Coll MA, Estruch Riba JSO. Double‐blind study of the efficacy of nicotine chewing gum for smoking cessation in the primary care setting [Estudio doble ciego de la eficacia del chicle de nicotina en la deshabituacion tabaquica dentro del ambito de la atencion primaria]. Atencion Primaria 1989;6(10):719‐26. - PubMed
Garvey 2000 {published data only}
-
- Doherty K, Militello FS, Kinnunen T, Garvey AJ. Nicotine gum dose and weight gain after smoking cessation. Journal of Consulting and Clinical Psychology 1996;64:799‐807. - PubMed
-
- Ferguson SG, Shiffman S, Rohay JM, Gitchell JG, Garvey AJ. Effect of compliance with nicotine gum dosing on weight gained during a quit attempt. Addiction 2011;106:651‐6. - PubMed
-
- Garvey AJ, Kinnunen T, Nordstrom BL, Utman CH, Doherty K, Rosner B, et al. Effects of nicotine gum dose by level of nicotine dependence. Nicotine & Tobacco Research 2000;2:53‐63. - PubMed
-
- Kinnunen T, Doherty K, Militello FS, Garvey AJ. Depression and smoking cessation ‐ characteristics of depressed smokers and effects of nicotine replacement. Journal of Consulting and Clinical Psychology 1996;64:791‐98. - PubMed
-
- Nordstrom BL, Kinnunen T, Utman CH, Garvey AJ. Long‐term effects of nicotine gum on weight gain after smoking cessation. Nicotine & Tobacco Research 1999;1:259‐68. - PubMed
Gilbert 1989 {published data only}
-
- Gilbert JR, Wilson DM, Best JA, Taylor DW, Lindsay EA, Singer J, et al. Smoking cessation in primary care. A randomized controlled trial of nicotine‐bearing chewing gum. Journal of Family Practice 1989;28:49‐55. - PubMed
Glavas 2003a {published data only}
-
- Glavas D, Rumboldt M, Rumboldt Z. Smoking cessation with nicotine replacement therapy among health care workers: randomized double‐blind study. Croatian Medical Journal 2003;44:219‐24. - PubMed
Glavas 2003b {published data only}
-
- Glavas D, Rumboldt Z. Smoking cessation using the transdermal nicotine system [Odvikavanje od pusenja transdermalnim nikotinskim sustavom]. Lijecnicki Vjesnik 2003;125(1‐2):8‐12. - PubMed
Glover 2002 {published data only}
-
- Glover E, Glover P, Franzon M, Sullivan R, Cerullo C. Safety and efficacy of a nicotine sublingual tablet for smoking cessation [abstract]. Smoke Free 21st Century: 2nd European Conference on Tobacco or Health; 1999; Las Palmas de Gran Canaria. 1999.
-
- Glover ED, Franzon M, Sullivan CR, Cerullo CL. A nicotine sublingual tablet for treating tobacco dependence [Abstract]. Society for Research on Nicotine and Tobacco 3rd Europe Conference; 2001 September; Paris, France. 2001:48.
-
- Glover ED, Glover PN, Franzon M, Sullivan CR, Cerullo CC, Howell RM, et al. A comparison of a nicotine sublingual tablet and placebo for smoking cessation. Nicotine & Tobacco Research 2002;4:441‐50. - PubMed
-
- Glover ED, Glover PN, Franzon M, Sullivan R, Sullivan P, Howell R, et al. A nicotine sublingual tablet for smoking cessation: 6‐month data [Abstract]. 10th World Conference on Tobacco or Health; 1997 August 24‐28; Beijing, China. 1997.
Gourlay 1995 {published data only}
Graham 2017 {published data only}
Gross 1995 {published data only}
-
- Gross J, Johnson J, Sigler L, Stitzer ML. Dose effects of nicotine gum. Addictive Behaviors 1995;20:371‐81. - PubMed
Hall 1985 {published data only}
-
- Hall SM, Tunstall C, Rugg D, Jones R, Benowitz N. Nicotine gum and behavioral treatment in smoking cessation. Journal of Consulting and Clinical Psychology 1985;53:256‐8. - PubMed
Hall 1987 {published data only}
-
- Hall SM, Tunstall CD, Ginsberg D, Benowitz NL, Jones RT. Nicotine gum and behavioral treatment: a placebo controlled trial. Journal of Consulting and Clinical Psychology 1987;55:603‐5. - PubMed
Hall 1996 {published data only}
-
- Hall SM, Muñoz RF, Reus VI, Sees KL, Duncan C, Humfleet GL, et al. Mood management and nicotine gum in smoking treatment ‐ a therapeutic contact and placebo‐controlled study. Journal of Consulting and Clinical Psychology 1996;64(5):1003‐9. - PubMed
Hand 2002 {published data only}
Harackiewicz 1988 {published data only}
-
- Harackiewicz JM, Blair LW, Sansone C, Epstein JA, Stuchell RN. Nicotine gum and self‐help manuals in smoking cessation: an evaluation in a medical context. Addictive Behaviors 1988;13:319‐30. - PubMed
Hasan 2014 {published data only}
-
- Hasan FM, Zagarins SE, Pischke KM, Saiyed S, Bettencourt AM, Beal L, et al. Hypnotherapy is more effective than nicotine replacement therapy for smoking cessation: results of a randomized controlled trial. Complementary Therapies in Medicine 2014;22(1):1‐8. - PubMed
Hays 1999 {published data only}
-
- Hays JT, Croghan GA, Offord KP, Wolter TD, Nides MA, Davidson M. Over‐the‐Counter (OTC) transdermal nicotine patch therapy [abstract]. Journal of Addictive Diseases 1997;16:136.
-
- Hays JT, Croghan IT, Offord KP, Hurt RD, Schroeder DR, Wolter TD, et al. Over the counter 22mg nicotine patch therapy for smoking cessation: results from randomized double‐blind placebo‐controlled and open label trials. Society for Research on Nicotine and Tobacco 5th Annual Meeting; 1999; San Diego, CA, USA 1999. - PMC - PubMed
Herrera 1995 {published data only}
-
- Herrera N, Franco R, Herrera L, Partidas A, Rolando R, Fagerström KO. Nicotine gum, 2 and 4 mg, for nicotine dependence. A double‐blind placebo‐controlled trial within a behavior modification support program. Chest 1995;108:447‐51. - PubMed
Heydari 2012 {published data only}
-
- Heydari G, Talischi F, Tafti SF, Masjedi MR. Quitting smoking with varenicline: parallel, randomised efficacy trial in Iran. International Journal of Tuberculosis and Lung Disease 2012;16(2):268‐72. [CENTRAL: 814663; CRS: 9400123000012712; EMBASE: 2012026893; PUBMED: 22236931] - PubMed
Heydari 2013 {published data only}
-
- Heydari G, Talischi F, Batmanghelidj E, Pajooh MR, Boroomand A, Zamani M, et al. Dual addictions, parallel treatments: Nicotine replacement therapy for patients receiving methadone treatment in the Islamic Republic of Iran. La Revue de Santé de la Mediterranée Orientale / al‐Majallah al‐sihhiyah li‐sharq al‐mutawassit (Eastern Mediterranean Health Journal) 2013;19(SUPPL 3):S25‐31. - PubMed
Hjalmarson 1984 {published data only}
-
- Hjalmarson AI. Effect of nicotine chewing gum in smoking cessation. A randomized, placebo‐controlled, double‐blind study. JAMA 1984;252:2835‐8. - PubMed
Hjalmarson 1994 {published data only}
-
- Hjalmarson AI, Franzon M, Westin A, Wiklund O. Effect of nicotine nasal spray on smoking cessation. A randomized, placebo‐controlled, double‐blind study. Archives of Internal Medicine 1994;154:2567‐72. - PubMed
Hjalmarson 1997 {published data only}
-
- Hjalmarson A, Nilsson F, Sjöström L, Wiklund O. The nicotine inhaler in smoking cessation. Archives of Internal Medicine 1997;157(15):1721‐8. - PubMed
Huber 1988 {published data only}
-
- Huber D. Combined and separate treatment effects of nicotine chewing gum and self‐control method. Pharmacopsychiatry 1988;21:461‐2. - PubMed
Hughes 1989a {published data only}
Hughes 1990 {published data only}
-
- Hughes JR, Gust SW, Keenan RM, Fenwick JW. Effect of dose on nicotine's reinforcing, withdrawal‐suppression and self‐reported effects. Journal of Pharmacology and Experimental Therapeutics 1990;252:1175‐83. - PubMed
Hughes 1999 {published data only}
-
- Hughes JR, Lesmes GR, Hatsukami DK, Richmond RL, Lichtenstein E, Jorenby DE, et al. Are higher doses of nicotine replacement more effective for smoking cessation?. Nicotine & Tobacco Research 1999;1:169‐74. - PubMed
Hughes 2003 {published data only}
-
- Hughes JR, Novy P, Hatsukami DK, Jensen J, Callas PW. Efficacy of nicotine patch in smokers with a history of alcoholism. Alcoholism‐Clinical and Experimental Research 2003;27:946‐54. - PubMed
Hurt 1990 {published data only}
-
- Elan Pharmaceutical Research Corp. NDA 19‐983 for Approval of PROSTEP. Study 88‐02 1992.
-
- Hurt RD, Lauger GG, Offord KP, Kottke TE, Dale LC. Nicotine‐replacement therapy with use of a transdermal nicotine patch‐a randomized double‐blind placebo‐controlled trial. Mayo Clinic Proceedings 1990;65:1529‐37. - PubMed
Hurt 1994 {published data only}
-
- Hurt RD, Dale LC, Fredrickson PA, Caldwell CC, Lee GA, Offord KP, et al. Nicotine patch therapy for smoking cessation combined with physician advice and nurse follow‐up: One‐year outcome and percentage of nicotine replacement. JAMA 1994;271:595‐600. - PubMed
ICRF 1994 {published data only}
-
- Johnstone EC, Yudkin Pl, Hey K, Roberts SJ, Welch SJ, Murphy MF, et al. Genetic variation in dopaminergic pathways and short‐term effectiveness of the nicotine patch. Pharmacogenetics 2004;14(2):83‐90. - PubMed
Jamrozik 1984 {published data only}
Jarvis 1982 {published data only}
Jensen 1991 {published data only}
-
- Jensen EJ, Schmidt E, Pedersen B, Dahl R. Effect on smoking cessation of silver acetate, nicotine and ordinary chewing gum. Influence of smoking history. Psychopharmacology (Berl) 1991;104:470‐4. - PubMed
-
- Jensen EJ, Schmidt E, Pedersen B, Dahl R. The effect of nicotine, silver acetate, and placebo chewing gum on the cessation of smoking. The influence of smoking type and nicotine dependence. International Journal of the Addictions 1991;26:1223‐31. - PubMed
Johns 2017 {published data only}
-
- Johns D. Randomised controlled trial comparing nicotine replacement therapy (nrt) and counselling on smoking cessation in patients prone to lung cancer using bed font micro‐smokerlyzer. International MASCC/ISOO Symposium: Supportive Care in Cancer; 2017; USA. 2017:S102.
Jorenby 1999 {published data only}
-
- Durcan MJ, White J, Jorenby DE, Fiore MC, Rennard SI, Leischow SJ, et al. Impact of prior nicotine replacement therapy on smoking cessation efficacy. American Journal of Health Behaviors 2002;26:213‐20. - PubMed
-
- Jamerson BD, Nides M, Jorenby DE, Donahue R, Garrett P, Johnston JA, et al. Late‐term smoking cessation despite initial failure: an evaluation of bupropion sustained release, nicotine patch, combination therapy, and placebo. Clinical Therapeutics 2001;23:744‐52. - PubMed
-
- Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, et al. A controlled trial of sustained‐release bupropion, a nicotine patch, or both for smoking cessation. New England Journal of Medicine 1999;340:685‐91. - PubMed
Joseph 1996 {published data only}
-
- Joseph AM, Antonnucio DO. Lack of efficacy of transdermal nicotine in smoking cessation. New England Journal of Medicine 1999;341:1157‐8. - PubMed
-
- Joseph AM, Norman SM, Ferry LH, Prochazka AV, Westman EC, Steele BG, et al. The safety of transdermal nicotine as an aid to smoking cessation in patients with cardiac disease. New England Journal of Medicine 1996;335:1792‐8. - PubMed
Killen 1984 {published data only}
-
- Killen JD, Maccoby N, Taylor CB. Nicotine gum and self‐regulation training in smoking relapse prevention. Behavior Therapy 1984;15:234‐48.
Killen 1990 {published data only}
-
- Fortmann SP, Killen JD, Telch MJ, Newman B. Minimal contact treatment for smoking cessation. A placebo controlled trial of nicotine polacrilex and self‐directed relapse prevention: initial results of the Stanford Stop Smoking Project. JAMA 1988;260:1575‐80. - PubMed
-
- Killen JD, Fortmann SP, Newman B, Varady A. Evaluation of a treatment approach combining nicotine gum with self‐guided behavioral treatments for smoking relapse prevention. Journal of Consulting and Clinical Psychology 1990;58:85‐92. - PubMed
Killen 1997 {published data only}
-
- Killen JD, Fortmann SP, Davis L, Varady A. Nicotine patch and self‐help video for cigarette smoking cessation. Journal of Consulting and Clinical Psychology 1997;65:663‐72. - PubMed
Kornitzer 1995 {published data only}
-
- Kornitzer M, Boutsen M, Dramaix M, Thijs J, Gustavsson G. Combined use of nicotine patch and gum in smoking cessation: a placebo‐controlled clinical trial. Preventive Medicine 1995;24:41‐7. - PubMed
-
- Kornitzer M, Boutsen M, Thijs J, Gustavsson G. Efficiency and safety of combined use of nicotine patches and nicotine gum in smoking cessation: a placebo controlled double‐blind trial [abstract]. European Respiratory Journal 1993;6(Suppl 17):630S.
Kralikova 2009 {published and unpublished data}
-
- Kralikova E, Kozak J, Rasmussen T, Cort N. The clinical benefits of NRT‐supported smoking reduction. Nicotine & Tobacco Research 2002;4:243.
Leischow 1996a {published data only}
-
- Leischow SJ, Nilsson F, Franzon M, Hill A, Otte P, Merikle EP. Efficacy of the nicotine inhaler as an adjunct to smoking cessation. American Journal of Health Behavior 1996;20:364‐71.
Lerman 2015 {published data only}
-
- Lerman C, Schnoll RA, Hawk LW Jr, Cinciripini P, George TP, Wileyto EP, et al. Use of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: a randomised, double‐blind placebo‐controlled trial. Lancet Respiratory Medicine 2015;3(2):131‐8. - PMC - PubMed
-
- Rabin RA, Ashare RL, Schnoll RA, Cinciripini PM, Hawk LW Jr, Lerman C, et al. Does cannabis use moderate smoking cessation outcomes in treatment‐seeking tobacco smokers? Analysis from a large multi‐center trial. American Journal on Addictions/American Academy of Psychiatrists in Alcoholism and Addictions 2016;25(4):291‐6. - PubMed
Lewis 1998 {published data only}
-
- Lewis SF, Piasecki TM, Fiore MC, Anderson JE, Baker TB. Transdermal nicotine replacement for hospitalized patients: A randomized clinical trial. Preventive Medicine 1998;27(2):296‐303. - PubMed
Llivina 1988 {published data only}
-
- Salvador Llivina T, Marin Tuya D, Gonzalez Quintana J, Iniesta Torres C, Castellvi Barrera E, Muriana Saez C, et al. Treatment of smoking: efficacy of the use of nicotine chewing gum. Double‐blind study [Tratamiento del tabaquismo: eficacia de la utilizacion del chicle de nicotina. Estudio a doble ciego]. Medicina Clinica Barcelona 1988;90:646‐50. - PubMed
Malcolm 1980 {published data only}
-
- Malcolm RE, Sillett RW, Turner JA, Ball KP. The use of nicotine chewing gum as an aid to stopping smoking. Psychopharmacology Series 1980;70:295‐6. - PubMed
McGovern 1992 {published data only}
-
- McGovern PG, Lando HA. An assessment of nicotine gum as an adjunct to Freedom from Smoking cessation clinics. Addictive Behaviors 1992;17:137‐47. - PubMed
Molyneux 2003 {published data only}
Moolchan 2005 {published data only}
-
- Franken FH, Pickworth WB, Epstein DH, Moolchan ET. Smoking rates and topography predict adolescent smoking cessation following treatment with nicotine replacement therapy. Cancer Epidemiology, Biomarkers & Prevention 2006;15:154‐7. - PubMed
-
- Jaszyna‐Gasior M, Schroeder JR, Thorner ED, Heishman SJ, Collins CC, Lo S, et al. Age at menarche and weight concerns in relation to smoking trajectory and dependence among adolescent girls enrolled in a smoking cessation trial. Addictive Behaviors 2009;34(1):92‐5. - PubMed
-
- Moolchan ET, Robinson ML, Ernst M, Cadet JL, Pickworth WB, Heishman SJ, et al. Safety and efficacy of the nicotine patch and gum for the treatment of adolescent tobacco addiction. Pediatrics 2005;115(4):e407‐e414. - PubMed
-
- Robinson ML, Schroeder JR, Moolchan ET. Adolescent smokers screened for a nicotine replacement treatment trial: Correlates of eligibility and enrollment. Nicotine & Tobacco Research 2006;8:447‐54. - PubMed
Mori 1992 {unpublished data only}
-
- Mori T, Shimao T, Yulchiro G, Namiki M, Hyachi T. A clinical trial of nicotine chewing gum for smoking cessation [abstract 428]. 8th World Conference on Tobacco or Health; 1992; Buenos Aires, Argentina. 1992.
Nakamura 1990 {unpublished data only}
-
- Nakamura M, Saito J, Oshima A, Miyamoto M, Matushita A, Endo S. Effect of nicotine chewing gun in smoking cessation classes. The Global War. Proceedings of the 7th World Conference on Tobacco and Health; 1990; Perth, Western Australia. Perth: Health Department of Western Australia, 1990:665‐7.
NCT00534404 {published data only}
-
- NCT00534404. iQuit Smoking: a randomized trial of internet access to nicotine patches. clinicaltrials.gov/show/NCT00534404 (first received 24 September 2007).
Nebot 1992 {published data only}
-
- Nebot M, Cabezas C. Does nurse counseling or offer of nicotine gum improve the effectiveness of physician smoking‐cessation advice?. Family Practice Research Journal 1992;12:263‐70. - PubMed
Niaura 1994 {published data only}
-
- Niaura R, Goldstein MG, Abrams DB. Matching high and low‐dependence smokers to self‐help treatment with or without nicotine replacement. Preventive Medicine 1994;23:70‐7. - PubMed
Niaura 1999 {published data only}
-
- Niaura R, Abrams DB, Shadel WG, Rohsenow DJ, Monti PM, Sirota AD. Cue exposure treatment for smoking relapse prevention: A controlled clinical trial. Addiction 1999;94(5):685‐96. - PubMed
Ockene 1991 {published data only}
-
- Ockene JK, Kristeller J, Goldberg R, Amick TL, Pekow PS, Hosmer D, et al. Increasing the efficacy of physician‐delivered smoking interventions: a randomized clinical trial. Journal of General Internal Medicine 1991;6:1‐8. - PubMed
-
- Ockene JK, Kristeller J, Pbert L, Hebert JR, Luippold R, Goldberg RJ, et al. The physician‐delivered smoking intervention project: can short‐term interventions produce long‐term effects for a general outpatient population?. Health Psychology 1994;13:278‐81. - PubMed
Oncken 2007 {published data only}
-
- Oncken C, Cooney J, Feinn R, Lando H, Kranzler HR. Transdermal nicotine for smoking cessation in postmenopausal women. Addictive Behaviors 2007;32:296‐309. - PubMed
-
- Oncken C, Cooney J, Lando H, Feinn R, Kranzler H. Transdermal nicotine for smoking cessation in postmenopausal women (POS1‐045). Society for Research on Nicotine and Tobacco 11th Annual Meeting; 2005; Prague, Czech Republic. 2005.
Oncken 2008 {published data only}
Ortega 2011 {published and unpublished data}
-
- Ortega F, Vellisco A, Márquez E, López‐Campos JL, Rodríguez A, Ángeles Sánchez M, et al. Effectiveness of a cognitive orientation program with and without nicotine replacement therapy in stopping smoking in hospitalised patients. Archivos de Bronconeumologia 2011;47(1):3‐9. - PubMed
-
- Valencia B, Ortega F, Vellisco A, Márquez‐Martín E, Campos JLL, Rodríguez AM, et al. Effectiveness of a cognitive orientation program with and without nicotine replacement therapy in stopping smoking in hospitalised patients [Abstract]. European Respiratory Journal 2011;38(55):775s [P4224]. - PubMed
Otero 2006 {published data only}
-
- Otero UB, Perez CA, Szklo M, Esteves GA, dePinho MM, Szklo AS, et al. Randomized clinical trial: effectiveness of the cognitive‐behavioral approach and the use of nicotine replacement transdermal patches for smoking cessation among adults in Rio de Janeiro, Brazil [Ensaio clinico randomizado: efetividade da abordagem cognitivo‐comportamental e uso de adesivos transdermicos de reposicao de nicotina, na cessacao de fumar, em adultos residentes no Municipio do Rio de Janeiro, Brasil]. Cadernos de Saude Publica 2006;22:439‐49. - PubMed
Page 1986 {published data only}
-
- Page AR, Walters DJ, Schlegel RP, Best JA. Smoking cessation in family practice: the effects of advice and nicotine chewing gum prescription. Addictive Behaviors 1986;11:443‐6. - PubMed
Paoletti 1996 {published data only}
-
- Cosci F, Corlando A, Fornai E, Pistelli F, Paoletti P, Carrozzi L. Nicotine dependence, psychological distress and personality traits as possible predictors of smoking cessation. Results of a double‐blind study with nicotine patch. Addictive Behaviors 2009;34(1):28‐35. - PubMed
-
- Fornai E, Desideri M, Pistelli F, Carrozzi L, Puntoni R, Avino S, et al. Smoking reduction in smokers compliant to a smoking cessation trial with nicotine patch. Monaldi Archives for Chest Disease 2001;56:5‐10. - PubMed
-
- Paoletti P, Fornai E, Maggiorelli F, Puntoni R, Viegi G, Carrozzi L, et al. Importance of baseline cotinine plasma values in smoking cessation: results from a double blind study with nicotine patch. European Respiratory Journal 1996;9:643‐51. - PubMed
Perng 1998 {published data only}
-
- Perng RP, Hsieh WC, Chen YM, Lu CC, Chiang SJ. Randomized, double‐blind, placebo‐controlled study of transdermal nicotine patch for smoking cessation. American Journal of Respiratory and Critical Care Medicine 1999;159(3SS):A735. - PubMed
-
- Perng RP, Hsieh WC, Chen YM, Lu CC, Chiang SJ. Randomized, double‐blind, placebo‐controlled study of transdermal nicotine patch for smoking cessation. Journal of the Formosan Medical Association 1998;97:547‐51. - PubMed
Piper 2009 {published data only}
Pirie 1992 {published data only}
Pollak 2007 {published data only}
-
- Pollak KI, Oncken C, Lipkus IM, Peterson BL, Swamy GK, Pletsch PK, et al. Effectiveness of adding nicotine replacement therapy to cognitive behavioral therapy for smoking cessation in pregnant smokers: the Baby Steps trial (PA6‐3). Society for Research on Nicotine and Tobacco 13th Annual Meeting; 2007 February 21‐24; Austin, Texas, USA. 2007:25.
Prapavessis 2007 {published data only}
-
- Prapavessis H, Cameron L, Baldi JC, Robinson S, Borrie K, Harper T, et al. The effects of exercise and nicotine replacement therapy on smoking rates in women. Addictive Behaviors 2007;32:1416‐32. - PubMed
Puska 1979 {published data only}
-
- Puska P, Bjorkqvist S, Koskela K. Nicotine‐containing chewing gum in smoking cessation: a double blind trial with half year follow‐up. Addictive Behaviors 1979;4:141‐6. - PubMed
Richmond 1993 {published data only}
-
- Richmond R, Heather N. General Practitioner interventions for smoking cessation: past results and future prospects. Behaviour Change 1990;7:110‐9.
-
- Richmond RL, Makinson RJ, Giugni AA, Webster IW. General Practitioner smoking interventions in Australia: results of studies over the past ten years. The Global War: Proceedings of the 7th World Conference on Tobacco and Health; 1990; Perth, Western Australia. Perth: Health Department of Western Australia, 1990:657‐60.
-
- Richmond RL, Makinson RJ, Kehoe LA, Giugni AA, Webster IW. One‐year evaluation of three smoking cessation interventions administered by general practitioners. Addictive Behaviors 1993;18:187‐99. - PubMed
Richmond 1994 {published data only}
-
- Richmond RL, Harris K, Almeida Neto A. The transdermal nicotine patch: results of a randomised placebo‐controlled trial. Medical Journal of Australia 1994;161:130‐5. - PubMed
-
- Richmond RL, Kehoe L, Neto ACdA. Effectiveness of a 24‐hour transdermal nicotine patch in conjunction with a cognitive behavioural programme: One year outcome. Addiction 1997;92:27‐31. - PubMed
Roto 1987 {published data only}
-
- Roto P, Ojala A, Sundman K, Jokinen K, Peltomakl R. Nicotine gum and withdrawal from smoking. Suomen Laakarllehtl 1987;36:3445‐8.
Russell 1983 {published data only}
Sachs 1993 {published data only}
-
- Sachs DPL, Sawe U, Leischow SJ. Effectiveness of a 16‐hour transdermal nicotine patch in a medical practice setting, without intensive group counseling. Archives of Internal Medicine 1993;153:1881‐90. - PubMed
Scherphof 2014 {published data only}
-
- Scherphof CS, Eijnden RJ, Engels RCME, Vollebergh WA. Short‐term efficacy of nicotine replacement therapy for smoking cessation in adolescents: a randomized controlled trial. Journal of Substance Abuse Treatment 2014;46(2):120‐7. - PubMed
-
- Scherphof CS, Eijnden RJ, Lugtig P, Engels RCME, Vollebergh WA. Adolescents' use of nicotine replacement therapy for smoking cessation: predictors of compliance trajectories. Psychopharmacology 2014;231(8):1743‐52. - PubMed
-
- Scherphof CS, Eijnden RJJM, Engels RCME, Vollebergh WAM. Long‐term efficacy of nicotine replacement therapy for smoking cessation in adolescents: A randomized controlled trial. Drug and Alcohol Dependence 2014;140:217‐20. - PubMed
Schneider 1983a {published data only}
-
- Jarvik ME, Schneider NG. Degree of addiction and effectiveness of nicotine gum therapy for smoking. American Journal of Psychiatry 1984;141:790‐1. - PubMed
-
- Schneider NG, Jarvik ME. Nicotine gum vs. placebo gum: comparisons of withdrawal symptoms and success rates. NIDA Research Monograph 1985;53:83‐101. - PubMed
-
- Schneider NG, Jarvik ME, Forsythe AB, Read LL, Elliott ML, Schweiger A. Nicotine gum in smoking cessation: a placebo‐controlled, double‐blind trial. Addictive Behaviors 1983;8:253‐61. - PubMed
Schneider 1983b {published data only}
-
- Schneider NG, Jarvik ME. Nicotine gum vs. placebo gum: comparisons of withdrawal symptoms and success rates. NIDA Research Monograph 1985;53:83‐101. - PubMed
-
- Schneider NG, Jarvik ME, Forsythe AB, Read LL, Elliott ML, Schweiger A. Nicotine gum in smoking cessation: a placebo‐controlled, double‐blind trial. Addictive Behaviors 1983;8:253‐61. - PubMed
Schneider 1995 {published data only}
-
- Schneider NG, Olmstead R, Mody FV, Doan K, Franzon M, Jarvik ME, et al. Efficacy of a nicotine nasal spray in smoking cessation: a placebo‐controlled, double‐blind trial. Addiction 1995;90:1671‐82. - PubMed
Schneider 1996 {published data only}
-
- Schneider NG, Olmstead R, Nilsson F, Mody FV, Franzon M, Doan K. Efficacy of a nicotine inhaler in smoking cessation: a double‐blind, placebo controlled trial. Addiction 1996;91:1293‐306. - PubMed
-
- Schneider NG, Olmstead R, Nilsson F, Mody FV, Franzon M, Doan K. Efficacy of a nicotine inhaler in smoking cessation: a double‐blind, placebo‐controlled trial [abstract]. Addiction 1997;92:630. - PubMed
Schnoll 2010 {published data only}
Segnan 1991 {published data only}
-
- Segnan N, Ponti A, Battista RN, Senore C, Rosso S, Shapiro SH, et al. A randomized trial of smoking cessation interventions in general practice in Italy. Cancer Causes and Control 1991;2:239‐46. - PubMed
-
- Senore C, Battista RN, Ponti A, Segnan N, Shapiro SH, Rosso S, et al. Comparing participants and nonparticipants in a smoking cessation trial: Selection factors associated with general practitioner recruitment activity. Journal of Clinical Epidemiology 1999;52:83‐9. - PubMed
-
- Senore C, Battista RN, Shapiro SH, Segnan N, Ponti A, Rosso S, et al. Predictors of smoking cessation following physicians' counseling. Preventive Medicine 1998;27:412‐21. - PubMed
Shiffman 2002 (2 mg) {published data only}
-
- Dresler CM, Shiffman S, Strahs KR. Safety profile of the new nicotine polacrilex lozenge (PO1 36). Society for Research on Nicotine and Tobacco 8th Annual Meeting: 2002; Savannah, Georgia, USA. 2002.
-
- Ferguson SG, Gitchell JG, Shiffman S. Continuing to wear nicotine patches after smoking lapses promotes recovery of abstinence. Addiction (Abingdon, England) 2012;107(7):1349‐53. - PubMed
-
- Shiffman S. Nicotine lozenge efficacy in light smokers. Drug & Alcohol Dependence 2005;77:311‐4. - PubMed
-
- Shiffman S. Use of more nicotine lozenges leads to better success in quitting smoking. Addiction 2007;102:809‐14. - PubMed
-
- Shiffman S, Dresler CM, Hajek P, Gilburt SJ, Targett DA, Strahs KR. Efficacy of a nicotine lozenge for smoking cessation. Archives of Internal Medicine 2002;162:1267‐76. - PubMed
Shiffman 2002 (4 mg) {published data only}
-
- Ferguson SG, Gitchell JG, Shiffman S. Continuing to wear nicotine patches after smoking lapses promotes recovery of abstinence. Addiction (Abingdon, England) 2012;107(7):1349‐53. - PubMed
-
- Shiffman S, Dresler CM, Hajek P, Gilburt SJ, Targett DA, Strahs KR. Efficacy of a nicotine lozenge for smoking cessation. Archives of Internal Medicine 2002;162:1267‐76. - PubMed
Shiffman 2009 (2 mg) {published data only}
-
- Shiffman S, Ferguson SG, Strahs KR. Quitting by gradual smoking reduction using nicotine gum: a randomized controlled trial. American Journal of Preventive Medicine 2009;36(2):96‐104. - PubMed
Shiffman 2009 (4 mg) {published data only}
-
- Shiffman S, Ferguson SG, Strahs KR. Quitting by gradual smoking reduction using nicotine gum: a randomized controlled trial. American Journal of Preventive Medicine 2009;36(2):96‐104. - PubMed
Stapleton 1995 {published data only}
-
- Stapleton JA, Russell MAH, Feyerabend C, Wiseman SM, Gustavsson G, Sawe U, et al. Dose effects and predictors of outcome in a randomized trial of transdermal nicotine patches in general practice. Addiction 1995;90:31‐42. - PubMed
Stein 2013 {published and unpublished data}
-
- NCT00790569. Varenicline versus nicotine replacement for methadone‐maintained smokers. ClinicalTrials.gov/ct2/ (first received 13 November 2008). [CRS: 9400123000011408]
-
- Stein MD, Caviness CM, Kurth ME, Audet D, Olson J, Anderson BJ. Varenicline for smoking cessation among methadone‐maintained smokers: A randomized clinical trial. Drug and Alcohol Dependence 2013;133(2):486‐93. [CENTRAL: 870955; CRS: 9400107000001457; EMBASE: 2013694276; PUBMED: 23953658] - PMC - PubMed
Sutherland 1992 {published data only}
-
- Sutherland G, Stapleton JA, Russell MAH, Jarvis MJ, Hajek P, Belcher M, et al. Randomised controlled trial of nasal nicotine spray in smoking cessation. Lancet 1992;340:324‐9. - PubMed
Sønderskov 1997 {published data only}
-
- Sønderskov J, Olsen J, Meillier L, Overvad OK, Sabroe S. The effect of transdermal nicotine patches in smoking cessation. A randomized trial in pharmacy customers in Denmark. Ugeskrift for Laeger 1999;161:593‐7. - PubMed
-
- Sønderskov J, Olsen J, Sabroe S, Meillier L, Overvad K. Nicotine patches in smoking cessation: A randomized trial among over‐ the‐counter customers in Denmark. American Journal of Epidemiology 1997;145:309‐18. - PubMed
TNSG 1991 {published data only}
-
- Daughton DM, Fortmann SP, Glover ED, Hatsukami DK, Heatley SA, Lichtenstein E, et al. The smoking cessation efficacy of varying doses of nicotine patch delivery systems 4 to 5 years post‐quit day. Preventive Medicine 1999;28:113‐8. - PubMed
-
- Ferguson SG, Gitchell JG, Shiffman S, Sembower MA. Prediction of abstinence at 10 weeks based on smoking status at 2 weeks during a quit attempt: secondary analysis of two parallel, 10‐week, randomized, double‐blind, placebo‐controlled clinical trials of 21‐mg nicotine patch in adult smokers. Clinical Therapeutics 2009;31(9):1957‐65. - PubMed
-
- Swan GE, Jack LM, Ward MM. Subgroups of smokers with different success rates after use of transdermal nicotine. Addiction 1997;92:207‐17. - PubMed
-
- Transdermal Nicotine Study Group. Transdermal nicotine for smoking cessation. Six‐month results from two multicenter controlled clinical trials. Transdermal Nicotine Study Group. JAMA 1991;266:3133‐8. - PubMed
Tuisku 2016 {published data only}
-
- Tuisku A, Salmela M, Nieminen P, Toljamo T. Varenicline and nicotine patch therapies in young adults motivated to quit smoking: a randomized, placebo‐controlled, prospective Studys. Basic & Clinical Pharmacology & Toxicology 2016;119(1):78‐84. - PubMed
Tønnesen 1988 {published data only}
-
- Tønnesen P, Fryd V, Hansen M, Helsted J, Gunnersen AB, Forchammer H, et al. Effect of nicotine chewing gum in combination with group counseling on the cessation of smoking. New England Journal of Medicine 1988;318:15‐8. - PubMed
Tønnesen 1991 {published data only}
-
- Mikkelsen KL, Tønnesen P, Norregaard J. Three‐year outcome of two‐ and three‐year sustained abstainers from a smoking cessation study with nicotine patches. Journal of Smoking‐Related Disorders 1994;5:95‐100.
-
- Norregaard J, Tønnesen P, Petersen L. Predictors and reasons for relapse in smoking cessation with nicotine and placebo patches. Preventive Medicine 1993;22:261‐71. - PubMed
-
- Tønnesen P, Norregaard J, Sawe U. Two‐year outcome in a smoking cessation trial with a nicotine patch. Journal of Smoking‐Related Disorders 1992;3:241‐5.
-
- Tønnesen P, Norregaard J, Simonsen K, Sawe U. A double‐blind trial of a 16‐hour transdermal nicotine patch in smoking cessation. New England Journal of Medicine 1991;325:311‐5. - PubMed
-
- Tønnesen P, Norregaard J, Simonsen K, Sawe U. A double‐blind trial of nicotine patches in smoking cessation [En dobbeltblind undersogelse af nikotinplaster ved rygeafvaenning]. Ugeskrift for Laeger 1992;154:251‐4. - PubMed
Tønnesen 1993 {published data only}
-
- Tønnesen P, Norregaard J, Mikkelsen K, Jorgensen S, Nilsson F. A double‐blind trial of a nicotine inhaler for smoking cessation. JAMA 1993;269:1268‐71. - PubMed
Tønnesen 2000 {published data only}
-
- Tønnesen P, Mikkelsen KL. Smoking cessation with four nicotine replacement regimes in a lung clinic. European Respiratory Journal 2000;16:717‐22. - PubMed
Tønnesen 2006 {published data only}
-
- Pbert L. Nurse‐conducted smoking cessation in patients with COPD, using nicotine sublingual tablets and behavioral support. Chest 2006;130:314‐6. - PubMed
-
- Tonnesen P, Mikkelsen K, Bremann L. Nurse‐conducted smoking cessation in patients with COPD using nicotine sublingual tablets and behavioral support. Chest 2006;130:334‐42. - PubMed
Tønnesen 2012 {published and unpublished data}
-
- Tønnesen P, Lauri H, Perfekt R, Mann K, Batra A. Efficacy and safety of a novel nicotine mouth spray in smoking cessation: A randomized, placebo‐controlled, double blind, multicenter study with 52‐week follow up. Poster Presented at SRNT, Feb 16‐19th, 2011, Toronto, Canada.
Villa 1999 {published data only}
-
- Villa RS, Alvarez ABD, Hermida JRF. Effectiveness of a multicomponent program to quit smoking with and without nicotine chewing gum [Eficacia de un programa multicomponente para dejar de fumar con y sin chicle de nicotina]. Psicologia Conductual 1999;7:107‐18.
Wallstrom 2000 {published data only}
-
- Pharmacia, Upjohn. Nicorette Nicotine Microtab Monograph. Chester: Adis International, 1998:Adis International, 1998.
-
- Wallstrom M, Nilsson F, Hirsch JM. A double‐blind placebo controlled clinical evaluation of a nicotine sublingual tablet in smoking cessation [abstract]. European Respiratory Journal 1997; Vol. 10, issue Suppl 25:440S. - PubMed
-
- Wallstrom M, Nilsson F, Hirsch JM. A randomized double‐blind placebo‐controlled clinical evaluation of a nicotine sublingual tablet in smoking cessation. Addiction 2000;95(8):1161‐71. - PubMed
Ward 2013 {published data only}
-
- Ben Taleb Z, Ward KD, Asfar T, Jaber R, Auf R, Maziak W. Predictors of nicotine withdrawal symptoms: findings from the first randomized smoking cessation trial in a low‐income country setting. International Journal of Public Health 2016;61(6):701‐8. - PubMed
-
- Ben Taleb Z, Ward KD, Asfar T, Jaber R, Bahelah R, Maziak W. Smoking cessation and changes in body mass index: Findings from the first randomized cessation trial in a low‐income country setting. Nicotine & Tobacco Research 2017;19(3):351‐6. - PubMed
-
- Ben Taleb Z, Ward KD, Asfar T, Jaber R, Bahelah R, Maziak W. Smoking cessation and changes in body mass index: findings from the first randomized cessation trial in a low‐Income country setting. Nicotine & Tobacco Research 2016;19(3):351‐56. - PubMed
-
- Lando H. Commentary on Ward et al. (2013): failure to find a treatment effect for NRT in a low‐income country ‐ implications. Addiction (Abingdon, England) 2013;108(2):404‐5. - PubMed
Wennike 2003b {published data only}
-
- Wennike P, Danielsson T, Landfeldt B, Westin A, Tønnesen P. Smoking reduction promotes smoking cessation: results from a double blind, randomized, placebo‐controlled trial of nicotine gum with 2‐year follow‐up. Addiction 2003;98(10):1395‐402. - PubMed
Westman 1993 {published data only}
-
- Westman EC, Levin ED, Rose JE. The nicotine patch in smoking cessation. A randomized trial with telephone counseling. Archives of Internal Medicine 1993;153:1917‐23. - PubMed
Wisborg 2000 {published data only}
-
- Wisborg K, Henriksen TB, Jespersen LB, Secher NJ. Nicotine patches for pregnant smokers: A randomized controlled study. Obstetrics & Gynecology 2000;96(6):967‐71. - PubMed
Wittchen 2011 {published data only}
Zelman 1992 {published data only}
-
- Zelman DC, Brandon TH, Jorenby DE, Baker TB. Measures of affect and nicotine dependence predict differential response to smoking cessation treatments. Journal of Consulting and Clinical Psychology 1992;60:943‐52. - PubMed
References to studies excluded from this review
Adelman 2009 {published data only}
-
- Adelman WP. Nicotine nasal spray neither effective nor well‐tolerated by adolescent smokers. Journal of Pediatrics 2009;154(3):462‐3. - PubMed
Allen 2005 {published data only}
-
- Allen SS, Hatsukami D, Brintnell DM, Bade T. Effect of nicotine replacement therapy on post‐cessation weight gain and nutrient intake: A randomized controlled trial of postmenopausal female smokers. Addictive Behaviors 2005;30:1273‐80. - PubMed
-
- Allen SS, Hatsukami DK, Bade T, Center B. Transdermal nicotine use in postmenopausal women: does the treatment efficacy differ in women using and not using hormone replacement therapy?. Nicotine & Tobacco Research 2004;6(5):777‐88. - PubMed
Allen 2011 {published data only}
-
- Allen MH, Debanne M, Lazignac C, Adam E, Dickinson LM, Damsa C. Effect of nicotine replacement therapy on agitation in smokers with schizophrenia: a double‐blind, randomized, placebo‐controlled study. American Journal of Psychiatry 2011;168(4):395‐9. - PubMed
Aubin 2006 {published data only}
-
- Aubin HJ, Luthringer R, Demazieres A, Dupont C, Lagrue G. Comparison of the effects of a 24‐hour nicotine patch and a 16‐hour nicotine patch on smoking urges and sleep. Nicotine & Tobacco Research 2006;8:193‐201. - PubMed
Batra 2005 {published data only}
-
- Batra A, Klingler K, Landfeldt B, Friederich HM, Westin A, Danielsson T. Smoking reduction treatment with 4‐mg nicotine gum: A double‐blind, randomized, placebo‐controlled study. Clinical Pharmacology & Therapeutics 2005;78:689‐96. - PubMed
-
- Landfeldt B, Batra A, Friederich HM, Klingler K, Westin A. Smoking reduction with a 4 mg nicotine gum ‐ final results from a placebo‐controlled trial over 13 months. Society for Research on Nicotine and Tobacco 5th European Meeting; 2003 November 20‐22; Padua, Italy. 2003.
Berlin 2011 {published data only}
-
- Berlin I, Jacob N, Coudert M, Perriot J, Schultz L, Rodon N. Adjustment of nicotine replacement therapies according to saliva cotinine concentration: the ADONIS* trial‐a randomized study in smokers with medical comorbidities. Addiction 2011;106(4):833‐43. - PubMed
Bock 2010 {published data only}
Bolliger 2000a {published data only}
-
- Bolliger CT. Practical experiences in smoking reduction and cessation. Addiction 2000;95(1 S1):S19‐S24. - PubMed
-
- Bolliger CT, Zellweger JP, Danielsson T, Biljon X, Robidou A, Westin A, et al. Influence of long‐term smoking reduction on health risk markers and quality of life. Nicotine & Tobacco Research 2002;4:433‐9. - PubMed
Bolliger 2007 {published data only}
-
- Bolliger CT, Biljon X, Axelsson A. A nicotine mouth spray for smoking cessation: a pilot study of preference, safety and efficacy. Respiration 2007;74(2):196‐201. - PubMed
Brantmark 1973a {published data only}
-
- Brantmark B, Ohlin P, Westling H. Nicotine‐containing chewing gum as an anti‐smoking aid. Psychopharmacologia 1973;31:191‐200. - PubMed
Caldwell 2016 {published data only}
-
- Caldwell BO, Crane J. Combination nicotine metered dose inhaler and nicotine patch for smoking cessation: a randomized controlled trial. Nicotine & Tobacco Research 2016;18(10):1944‐51. - PubMed
Carpenter 2003 {published data only}
-
- Carpenter MJ, Hughes JR, Keely JP. Effect of smoking reduction on later cessation: a pilot experimental study. Nicotine & Tobacco Research 2003;5(2):155‐62. - PubMed
Carpenter 2011 {published data only}
Chan 2010 {published data only}
-
- Chan SS, Leung DY, Abdullah AS, Lo SS, Yip AW, Kok WM, et al. Smoking‐cessation and adherence intervention among Chinese patients with erectile dysfunction. American Journal of Preventive Medicine 2010;39(3):251‐8. - PubMed
Chan 2011 {published data only}
-
- Chan SS, Leung DY, Abdullah AS, Wong VT, Hedley AJ, Lam TH. A randomized controlled trial of a smoking reduction plus nicotine replacement therapy intervention for smokers not willing to quit smoking. Addiction 2011;106(6):1155‐63. - PubMed
Chou 2004 {published data only}
-
- Chou KR, Chen R, Lee JF, Ku CH, Lu RB. The effectiveness of nicotine‐patch therapy for smoking cessation in patients with schizophrenia. International Journal of Nursing Studies 2004;41:321‐30. - PubMed
Christen 1984 {published data only}
-
- Christen AG, Drook C, McDonald JL, Stookey G, Olson B. Efficacy of nicotine chewing gum in facilitating smoking cessation. Journal of the American Dental Association 1984;108:594‐7. - PubMed
Cohen 1989a {published data only}
-
- Cohen SJ, Stookey GK, Katz BP, Drook CA, Christen AG. Helping smokers quit: a randomized controlled trial with private practice dentists. Journal of the American Dental Association 1989;118:41‐5. - PubMed
Cohen 1989b {published data only}
-
- Cohen SJ, Stookey GK, Katz BP, Drook CA, Smith DM. Encouraging primary care physicians to help smokers quit. A randomised, controlled trial. Annals of Internal Medicine 1989;110:648‐52. - PubMed
Croghan 2007 {published data only}
-
- Clark MM, Hurt RD, Croghan I, Patten CA, Novotny P, Sloan JA, et al. The prevalence of weight concerns in a smoking abstinence clinical trial (POS2‐84). Society for Research on Nicotine and Tobacco 12th Annual Meeting; 2006 February 15‐18, Orlando, Florida, USA. 2006.
-
- Croghan IT, Hurt RD, Croghan GA, Sloan JA. Comparing nicotine inhaler, bupropion and nicotine inhaler plus bupropion in treating tobacco dependence [abstract]. Nicotine & Tobacco Research 2005;7(4):680‐1.
-
- Croghan IT, Hurt RD, Dakhil SR, Croghan GA, Sloan JA, Novotny PJ, et al. Randomized comparison of a nicotine inhaler and bupropion for smoking cessation and relapse prevention. Mayo Clinic Proceedings 2007;82:186‐95. - PubMed
Cummings 2011 {published data only}
-
- Cummings KM, Hyland A, Carlin‐Menter S, Mahoney MC, Willett J, Juster HR. Costs of giving out free nicotine patches through a telephone quit line. Journal of Public Health Management & Practice 2011;17(3):E16‐23. - PubMed
Dey 1999 {published data only}
Donny 2009 {published data only}
Ebbert 2009 {published data only}
Ebbert 2010 {published data only}
Elan Pharm 88‐02 {published data only}
-
- Elan Pharmaceutical Research Corp. NDA 19‐983 for Approval of PROSTEP. Study 88‐02 1992.
Elan Pharm 90‐03 {published data only}
-
- Elan Pharmaceutical Research Corp. NDA 19‐983 for Approval of PROSTEP. Study 90‐03 1992.
Etter 2004 {published data only}
-
- Dar R, Stronguin F, Etter JF. Assigned versus perceived placebo effects in nicotine replacement therapy for smoking reduction in Swiss smokers. Journal of Consulting & Clinical Psychology 2005;73:350‐3. - PubMed
-
- Etter JF, Laszlo E, Perneger TV. Postintervention effect of nicotine replacement therapy on smoking reduction in smokers who are unwilling to quit: Randomized trial. Journal of Clinical Psychopharmacology 2004;24(2):174‐9. - PubMed
-
- Etter JF, Laszlo E, Zellweger JP, Perrot C, Perneger TV. Nicotine replacement to reduce cigarette consumption in smokers who are unwilling to quit: a randomized trial. Journal of Clinical Psychopharmacology 2002;22:487‐95. - PubMed
Fagerström 1993 {published data only}
-
- Fagerström KO, Schneider NG, Lunell E. Effectiveness of nicotine patch and nicotine gum as individual versus combined treatments for tobacco withdrawal symptoms. Psychopharmacology (Berl) 1993;111:271‐7. - PubMed
Fagerström 1997 {published data only}
Fagerström 2000 {published data only}
Ferguson 2012 {published data only}
Finland unpublished {unpublished data only}
-
- Anon. Combination NRT; Improving efficacy in smoking cessation. McNeil Consumer Healthcare booklet, 2007. Short term outcomes reported on p17.
Foulds 1993 {published data only}
-
- Foulds J, Stapleton J, Hayward M, Russell MA, Feyerabend C, Fleming T, et al. Transdermal nicotine patches with low‐intensity support to aid smoking cessation in outpatients in a general hospital. A placebo‐controlled trial. Archives of Family Medicine 1993;2:417‐23. - PubMed
Garvey 2006 {unpublished data only}
-
- Garvey AJ, Hoskinson RA, Wadler BM, Kinnunen T, Sachs DPL. Individualising nicotine patch dose to match smokers' usual nicotine intake levels (PA9‐4). Society for Research on Nicotine and Tobacco 12th Annual Meeting; 2006 February 15‐18; Orlando, Florida, USA. 2006:32.
-
- Mustonen TK, Spencer SM, Hoskinson RA, Sachs DPL, Garvey AJ. The influence of gender, race, and menthol content on tobacco exposure measures. Nicotine & Tobacco Research 2005;7:581‐90. - PubMed
Glover 1992 {published data only}
-
- Glover ED, Glover PN, Sullivan CR, Sullivan P, Nilsson F, Sawe U. Nicotine inhaler versus placebo in smoking cessation [abstract 237]. 8th World Conference on Tobacco or Health; 1992; Buenos Aires, Argentina. 1992.
Gross 1989 {published data only}
-
- Gross J, Stitzer ML, Maldonado J. Nicotine replacement: effects on postcessation weight gain. Journal of Consulting and Clinical Psychology 1989;57:87‐92. - PubMed
Guo 2006 {published data only}
-
- Guo S, Sun H, Niu G, Gao J, Yang X, Zhou D. The smoking cessation efficacy of nicotine sublingual tablet: A double‐blind, placebo controlled trial. Chinese Journal of Psychiatry 2006;39(2):102‐5.
Hajek 1999 {published data only}
-
- Hajek P, West R, Foulds J, Nilsson F, Burrows S, Meadow A. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Archives of Internal Medicine 1999;159:2033‐8. - PubMed
-
- West R, Hajek P, Foulds J, Nilsson F, May S, Meadows A. A comparison of the abuse liability and dependence potential of nicotine patch, gum, spray and inhaler. Psychopharmacology (Berl) 2000;149:198‐202. - PubMed
Hanson 2003 {published data only}
-
- Hanson K, Allen S, Jensen S, Hatsukami D. Treatment of adolescent smokers with the nicotine patch. Nicotine & Tobacco Research 2003;5(4):515‐26. - PubMed
Haustein 2003 {published data only}
-
- Haustein KO, Batra A, Landfeldt B, Westin A. The effect of short‐term or long‐term reduction on smoking cessation; results from a placebo controlled smoking reduction study with the nicotine gum. Nicotine & Tobacco Research 2003;5:278.
-
- Pfizer. Summary of clinical efficacy. Application for licensing of Nicorette Inhalator/Gum for smoking reduction leading to cessation. Company data NICORE‐1013‐273‐SU.
Hoch 2006 {published data only}
-
- Hoch E, Wittchen HU. Population health perspective on smoking cessation: A randomized controlled trial of different methods in primary health care (RPOS 3‐71). Society for Research on Nicotine and Tobacco 12th Annual Meeting; 2006 February 15‐18; Orlando, Florida, USA. 2006.
-
- Sonntag H, Hoch E, Jahn B, Spiegel B, Pfister H, Wittchen HU. Smoking cessation in primary care: implementation effectiveness and optimized allocation. Suchtmedizin in Forschung und Praxis 2003;5(2):137‐41.
Hotham 2006 {published data only}
-
- Hotham ED, Gilbert AL, Atkinson ER. A randomised‐controlled pilot study using nicotine patches with pregnant women. Addictive Behaviors 2006;31:641‐8. - PubMed
Hughes 1989b {published data only}
-
- Hughes JR, Gulliver SB, Amori G, Mireault GC, Fenwick JF. Effect of instructions and nicotine on smoking cessation, withdrawal symptoms and self‐administration of nicotine gum. Psychopharmacology (Berl) 1989;99:486‐91. - PubMed
Hurt 1995 {published data only}
-
- Hurt RD, Dale LC, Offord KP, Croghan IT, Hays JT, Gomez Dahl L. Nicotine patch therapy for smoking cessation in recovering alcoholics. Addiction 1995;90(11):1541‐6. - PubMed
Hurt 2003 {published data only}
-
- Hurt RD, Krook JE, Croghan IT, Loprinzi CL, Sloan JA, Novotny PJ, et al. Nicotine patch therapy based on smoking rate followed by bupropion for prevention of relapse to smoking. Journal of Clinical Oncology 2003;21(5):914‐20. - PubMed
Jarvik 1984 {published data only}
-
- Jarvik ME, Schneider NG. Degree of addiction and effectiveness of nicotine gum therapy for smoking. American Journal of Psychiatry 1984;141:790‐1. - PubMed
Jibrail 2010 {published data only}
-
- Jibrail JJ, Cortas NK, Sarieddine DS, Kanj NA, Zaatari GS, Daher RT. Impact of nicotine metabolite monitoring on the efficacy of smoke cessation and usefulness of sequential CRP measurements. American Journal of Respiratory Critical Care Medicine 2010;181:A2652.
Kapur 2001 {published data only}
-
- Kapur B, Hackman R, Selby P, Klein J, Koren G. Randomized, double‐blind, placebo‐controlled trial of nicotine replacement therapy in pregnancy. Current Therapeutic Research Clinical and Experimental 2001;62:274‐8.
Korberly 1999 {unpublished data only}
-
- Korberly B, Gustavsson G, Kruse E. Over‐the‐counter efficacy of a 15 mg daytime nicotine patch for smoking cessation: a randomized multicenter trial. Society for Research on Nicotine and Tobacco 4th European Conference; 2002 October 03‐05; Santander, Spain. 2002:23.
-
- Korberly BH, Maguire MK. An open label multicenter trial to evaluate and compare the efficacy of nicotrol 15mg as part of an OTC intervention package or as a prescription as an aid in smoking cessation. Society for Research on Nicotine and Tobacco Fifth Annual Meeting; 1999;San Diego, CA, USA. 1999.
Kozak 1995 {published data only}
-
- Kozak J, Fagerström KO, Sawe U. High‐dose treatment with the nicotine patch. International Journal of Smoking Cessation 1995;4(2):26‐8.
Kras 2010 {published data only}
-
- Kras M, Stough C, Scholey A, Kure C, Camfield D. Hypericum perforatum, nicotine patches and combination hypericum perforatum/nicotine patches for smoking cessation. European Neuropsychopharmacology 2010;20:S608‐9.
Krumpe 1989 {published data only}
-
- Krumpe P, Malani N, Adler J. Efficacy of transdermal nicotine administration as an adjunct for smoking cessation in heavily nicotine addicted smokers. Annual Review of Respiratory Disease 1989;139:A337.
Krupski 2016 {published data only}
-
- Krupski L, Cummings KM, Hyland A, Mahoney MC, Toll BA, Carpenter MJ, et al. Cost and effectiveness of combination nicotine replacement therapy among heavy smokers contacting a quitline. Journal of Smoking Cessation 2016;11(1):50‐9.
Kupecz 1996 {published data only}
-
- Kupecz D, Prochazka A. A comparison of nicotine delivery systems in a multimodality smoking cessation program. Nurse Practitioner 1996;21(2):73, 77‐8, 81. - PubMed
Landfeldt 1998 {unpublished data only}
-
- Landfeldt B, Kruse E, Westin A, Mattson K, Lojander J. Nicotine replacement treatment in heavy smokers: nicotine nasal spray combined with nicotine patch in a double‐blind controlled study (abstract). European Respiratory Journal 1998;12(Suppl 28):154S.
Leischow 1996b {published data only}
-
- Leischow SJ, Hill A, Cook G. The effects of transdermal nicotine for the treatment of hispanic smokers. American Journal of Health Behavior 1996;20:304‐11.
Levin 1994 {published data only}
-
- Levin ED, Westman EC, Stein RM, Carnahan E, Sanchez M, Herman S, et al. Nicotine skin patch treatment increases abstinence, decreases withdrawal symptoms, and attenuates rewarding effects of smoking. Journal of Clinical Psychopharmacology 1994;14:41‐9. - PubMed
Lin 1996 {published data only}
-
- Lin HN. The effectiveness of nicotine patch for smoking cessation. Chinese Psychiatry 1996;10:29‐38.
Marsh 2005 {published data only}
-
- Marsh HS, Dresler CM, Choi JH, Targett DA, Gamble ML, Strahs KR. Safety profile of a nicotine lozenge compared with that of nicotine gum in adult smokers with underlying medical conditions: A 12‐week, randomized, open‐label study. Clinical Therapeutics 2005;27(10):1571‐87. - PubMed
McCarthy 2006 {published data only}
-
- McCarthy DE, Piasecki TM, Fiore MC, Baker TB. Life before and after quitting smoking: an electronic diary study. Journal of Abnormal Psychology 2006;115(3):454‐66. - PubMed
McRobbie 2010 {published data only}
-
- McRobbie H, Thornley S, Bullen C, Lin RB, Senior H, Laugesen M, et al. A randomized trial of the effects of two novel nicotine replacement therapies on tobacco withdrawal symptoms and user satisfaction. Addiction 2010;105(7):1290‐8. - PubMed
Meier 1990 {published data only}
-
- Meier Lammermann E, Mayer M, Boleski PL. Combination of transdermal nicotine substitution and behavioural group therapy in smoking cessation. European Respiratory Journal 1990;3(Suppl 10):168S.
Merz 1993 {published data only}
-
- Merz PG, Keller Stanislawski B, Huber T, Woodcock BG, Rietbrock N. Transdermal nicotine in smoking cessation and involvement of non‐ specific influences. International Journal of Clinical Pharmacology Therapy and Toxicology 1993;31:476‐82. - PubMed
Miller 2009 {published data only}
Millie 1989 {published data only}
-
- Millie A, Berriau T, Paget D, Philardeau V, Postal MJ, Dautzenberg B, et al. Can weight gain during weaning from smoking be limited using nicotine gum? [Peut‐on limiter la prise de poids chez les fumeurs lors du sevrage tabagique en utilisant la gomme a la nicotine?]. Revue de Pneumonologie Clinique 1989;45:243‐9. - PubMed
Minneker 1989 {published data only}
-
- Minneker E, Buchkremer G, Block M. The effect of different dosages of a transdermal nicotine substitution system on the success rate of smoking cessation therapy. Methods and Findings in Experimental and Clinical Pharmacology 1989;11:219‐22. - PubMed
Molander 2000 {published data only}
-
- Molander L, Lunell E, Fagerström KO. Reduction of tobacco withdrawal symptoms with a sublingual nicotine tablet: a placebo controlled study. Nicotine & Tobacco Research 2000;2:187‐91. - PubMed
Mooney 2005 {published data only}
-
- Mooney M, Babb D, Jensen J, Hatsukami D. Interventions to increase use of nicotine gum: A randomized, controlled, single‐blind trial. Nicotine & Tobacco Research 2005;7(4):565‐79. - PubMed
Mulligan 1990 {published data only}
-
- Mulligan SC, Masterson JG, Devane JG, Kelly JG. Clinical and pharmacokinetic properties of a transdermal nicotine patch. Clinical Pharmacology and Therapeutics 1990;47:331‐7. - PubMed
Nackaerts 2009 {published data only}
-
- Nackaerts K, Salhi B, Devolder A, Meysman M, Boudrez H, Van‐Dyck L. A randomised, double‐blind, placebo‐controlled phase 3 study investigating the efficacy of nicotine substitution (NS) on nicotine withdrawal symptoms (NWS) in hospitalised smokers (HS) [Abstract]. European Respiratory Society Annual Congress; 2009 September 12‐16; Vienna, Austria,. 2009:4632.
NCT00000437 {published data only}
-
- NCT00000437. Tobacco dependence in alcoholism treatment (nicotine patch/naltrexone). ClinicalTrials.gov/show/NCT00000437 (first received 3 November 1999).
Okuyemi 2007 {published data only}
-
- Okuyemi KS, James AS, Mayo MS, Nollen N, Catley D, Choi WS, et al. Pathways to health: a cluster randomized trial of nicotine gum and motivational interviewing for smoking cessation in low‐income housing. Health Education & Behavior 2007;34:43‐54. - PubMed
Oncken 2009 {published data only}
Piper 2016 {published data only}
Pomerleau 2003 {published data only}
-
- Lerman C, Audrain J, Patterson F, Kaufmann V, Rukstalis M, Wileyto EP, et al. Differential response to nicotine replacement therapies in obese and non‐obese women (PA2‐6). Society for Research on Nicotine and Tobacco 9th Annual Meeting; 2003; New Orleans, LA, USA. 2003.
-
- Pomerleau OF, Pomerleau CS, Marks JL, Snedecor SM, Mehringer AM, Namenek‐Brouwer RJ, et al. Prolonged nicotine patch use in quitters with past abstinence‐induced depressed mood. Journal of Substance Abuse Treatment 2003;24(1):13‐18. - PubMed
Rennard 2006 {published data only}
-
- Rennard SI, Glover E, Leischow S, Daughton DM, Glover P, Muramoto M. Efficacy of nicotine inhaler in smoking reduction. Nicotine & Tobacco Research 2002;4:380. - PubMed
-
- Rennard SI, Glover ED, Leischow S, Daughton DM, Glover PN, Muramoto M, et al. Efficacy of the nicotine inhaler in smoking reduction: A double‐blind, randomized trial. Nicotine & Tobacco Research 2006;8:555‐64. - PubMed
Rey 2009 {published data only}
Rigotti 2009 {published data only}
-
- Rigotti NA, Gonzales D, Dale LC, Lawrence D, Chang Y, CIRRUS Study Group. A randomized controlled trial of adding the nicotine patch to rimonabant for smoking cessation: efficacy, safety and weight gain. Addiction 2009;104(2):266‐76. - PubMed
Roddy 2006 {published data only}
Rose 1990 {published data only}
-
- Rose JE, Levin ED, Behm FM, Adivi C, Schur C. Transdermal nicotine facilitates smoking cessation. Clinical Pharmacology and Therapeutics 1990;47:323‐30. - PubMed
Rubinstein 2008 {published data only}
Sachs 1995 {published data only}
-
- Sachs DPL. Effectiveness of the 4‐mg dose of nicotine polacrilex for the initial treatment of high‐dependent smokers. Archives of Internal Medicine 1995;155:1973‐80. - PubMed
Schlam 2016 {published data only}
Schneider 2004 {published data only}
-
- Schneider NG, Olmstead RE, Nides M, Mody FV, Otte Colquette P, Doan K, et al. Comparative testing of 5 nicotine systems: initial use and preferences. American Journal of Health Behavior 2004;28(1):72‐86. - PubMed
Schneider 2008 {published data only}
-
- Schneider NG, Cortner C, Gould JL, Koury MA, Olmstead RE. Comparison of craving and withdrawal among four combination nicotine treatments. Human Psychopharmacology 2008;23(6):513‐7. - PubMed
Schnoll 2015 {published data only}
Shahab 2011 {published data only}
-
- Shahab L, McEwen A, West R. Acceptability and effectiveness for withdrawal symptom relief of a novel oral nicotine delivery device: a randomised crossover trial. Psychopharmacology 2011;216(2):187‐96. - PubMed
Shiffman 2000a {published data only}
-
- Shiffman S, Khayrallah M, Nowak R. Efficacy of the nicotine patch for relief of craving and withdrawal 7‐10 weeks after cessation. Nicotine & Tobacco Research 2000;2:371‐8. - PubMed
Shiffman 2000b {published data only}
-
- Shiffman S, Elash CA, Paton SM, Gwaltney CJ, Paty JA, Clark DB, et al. Comparative efficacy of 24‐hour and 16‐hour transdermal nicotine patches for relief of morning craving. Addiction 2000;95(8):1185‐95. - PubMed
Shiffman 2002a {published data only}
-
- Ferguson SG, Gitchell JG, Shiffman S. Continuing to wear nicotine patches after smoking lapses promotes recovery of abstinence. Addiction 2012;107(7):1349‐53. - PubMed
-
- Shiffman S, Dresler CM, Gorsline J, Dimarino ME. The efficacy of nicotine patch in an over‐the‐counter environment: results from a randomized, double‐blind, placebo‐controlled trial (PO130). 11th World Conference on Tobacco or Health; 2000 August 06‐11; Chicago, Illinois, USA. 2000; Vol. 1.
-
- Shiffman S, Gorsline J, Gorodetzky CW. Efficacy of over‐the‐counter nicotine patch. Nicotine & Tobacco Research 2002;4:477‐83. - PubMed
Shiffman 2002b {published data only}
-
- Shiffman S, Rolf CN, Hellebusch SJ, Gorsline J, Gorodetzky CW, Chiang YK, et al. Real‐world efficacy of prescription and over‐the‐counter nicotine replacement therapy. Addiction 2002;97:505‐16. - PubMed
Shiffman 2006 {published data only}
-
- Ferguson SG, Shiffman S. Effect of high‐dose nicotine patch on the characteristics of lapse episodes. Health Psychology 2010;29:358‐66. - PubMed
-
- Ferguson SG, Shiffman S, Gwaltney CJ. Does reducing withdrawal severity mediate nicotine patch efficacy? A randomized clinical trial. Journal of Consulting and Clinical Psychology 2006;74:1153‐61. - PubMed
-
- Shiffman S, Ferguson SG, Gwaltney CJ. Immediate hedonic response to smoking lapses: Relationship to smoking relapse, and effects of nicotine replacement therapy. Psychopharmacology 2006;184(3‐4):608‐18. - PubMed
-
- Shiffman S, Ferguson SG, Gwaltney CJ, Balabanis MH, Shadel WG. Reduction of abstinence‐induced withdrawal and craving using high‐dose nicotine replacement therapy. Psychopharmacology 2006;184(3‐4):637‐44. - PubMed
Stapleton 2011 {published data only}
-
- Stapleton JA, Sutherland G. Treating heavy smokers in primary care with the nicotine nasal spray: randomized placebo‐controlled trial. Addiction 2011;106(4):824‐32. - PubMed
Sun 2009 {published data only}
-
- Sun HQ, Guo S, Chen DF, Jiang ZN, Liu Y, Di XL, et al. Family support and employment as predictors of smoking cessation success: a randomized, double‐blind, placebo‐controlled trial of nicotine sublingual tablets in Chinese smokers. American Journal of Drug & Alcohol Abuse 2009;35(3):183‐8. - PubMed
Sussman 2004 {published data only}
Sutherland 1999 {published data only}
-
- Anon. Combination NRT; Improving efficacy in smoking cessation. McNeil Consumer Healthcare booklet. Short term outcomes reported on p18.
-
- Sutherland G. A placebo‐controlled double‐blind combination trial of nicotine spray and patch [abstract]. Nicotine & Tobacco Research 1999;1:186.
Sutherland 2005 {published data only}
-
- Sutherland G, Stapleton JA, Russell MA. Randomized placebo‐controlled trial of nicotine nasal spray in general practice. Nicotine & Tobacco Research 2005;7(4):686.
Sutton 1987 {published data only}
Sutton 1988 {published data only}
-
- Sutton S, Hallett R. Smoking intervention in the workplace using videotapes and nicotine chewing gum. Preventive Medicine 1988;17:48‐59. - PubMed
Thorsteinsson 2001 {published data only}
-
- Thorsteinsson HS, Gillin JC, Patten CA, Golshan S, Sutton LD, Drummond S, et al. The effects of transdermal nicotine therapy for smoking cessation on depressive symptoms in patients with major depression. Neuropsychopharmacology 2001;24(4):350‐8. - PubMed
Tsukahara 2010 {published data only}
-
- Tsukahara H, Noda K, Saku K. A randomized controlled open comparative trial of varenicline vs nicotine patch in adult smokers: efficacy, safety and withdrawal symptoms (the VN‐SEESAW study). Circulation Journal 2010;74(4):771‐8. - PubMed
Tundulawessa 2010 {published data only}
-
- Tundulawessa Y, Yongchaiyud P, Chutrthong W, Tundulawessa K. The bioequivalent and effect of nicotine formulation gum on smoking cessation. Journal of the Medical Association of Thailand 2010;93(5):574‐9. - PubMed
Tzivoni 1998 {published data only}
-
- Tzivoni D, Keren A, Meyler S, Khoury Z, Lerer T, Brunel P. Cardiovascular safety of transdermal nicotine patches in patients with coronary artery disease who try to quit smoking. Cardiovascular Drugs and Therapy 1998;12:239‐44. - PubMed
Tønnesen 1996 {published data only}
-
- Tønnesen P, Mikkelsen K, Norregaard J, Jorgensen S. Recycling of hard‐core smokers with nicotine nasal spray. European Respiratory Journal 1996;9:1619‐23. - PubMed
Uyar 2005 {unpublished data only}
-
- Uyar M, Bayram N, Filiz A, Elbek O, Topçu A, Dikensoy O, et al. Comparison of nicotine patch and bupropion in treating tobacco dependence. European Respiratory Journal 2005;26(Suppl 49):388s.
Velicer 2006 {published data only}
-
- Velicer WF, Friedman RH, Fava JL, Gulliver SB, Keller S, Sun X, et al. Evaluating nicotine replacement therapy and stage‐based therapies in a population‐based effectiveness trial. Journal of Consulting & Clinical Psychology 2006;74:1162‐72. - PubMed
-
- Velicer WF, Keller S, Friedman RH, Fava JL, Gulliver SB, Ward RM, et al. Comparing participants and nonparticipants recruited for an effectiveness study of nicotine replacement therapy. Annals of Behavioral Medicine 2005;29:181‐91. - PubMed
Vial 2002 {published data only}
-
- Vial RJ, Jones TE, Ruffin RE, Gilbert AL. Smoking cessation program using nicotine patches linking hospital to the community. Journal of Pharmacy Practice and Research 2002;32(1):57‐62.
Vikhireva 2003 {published data only}
-
- Vikhireva O, Shalnova S, Deev A. Nicotine replacement therapy in Russia: old wine in new skins? Randomized parallel study of nicotine gum/inhaler in smoking cessation/reduction. Society for Research on Nicotine and Tobacco. 5th European Conference; 2003 November 20‐22; Padua, Italy. 2003.
-
- Vikhireva O, Shalnova S, Deev A, Levshin V, Radkevich N, Kalinina A. NRT‐assisted cessation in Russia: individual and population level benefits. Society for Research on Nicotine and Tobacco 11th Annual Meeting; 2005 March 20‐23; Prague, Czech Republic. 2005.
Warner 2005 {published data only}
-
- Warner DO, Patten CA, Ames SC, Offord KP, Schroeder DR. Effect of nicotine replacement therapy on stress and smoking behavior in surgical patients. Anesthesiology 2005;102(6):1138‐46. - PubMed
Wennike 2003a {published data only}
-
- Wennike P, Danielsson T, Landfeldt B, Westin A, Tønnesen P. Smoking reduction promotes smoking cessation: results from a double blind, randomized, placebo‐controlled trial of nicotine gum with 2‐year follow‐up. Addiction 2003;98(10):1395‐402. - PubMed
Williams 2007 {published and unpublished data}
-
- Williams JM, Gandhi KK, Foulds J, Steinberg M, Lou S, Masumova F, et al. No advantage for high dose compared to regular dose nicotine patch on short‐term abstinence rates in schizophrenia (PA2‐3). Society for Research on Nicotine and Tobacco 13th Annual Meeting; 2007 February 21‐24, Austin, Texas. 2007.
Wiseman 2005 {published data only}
-
- Wiseman EJ, Williams DK, McMillan DE. Effectiveness of payment for reduced carbon monoxide levels and noncontingent payments on smoking behaviors in cocaine‐abusing outpatients wearing nicotine or placebo patches. Experimental and Clinical Psychopharmacology 2005;13(2):102‐10. - PubMed
Working Group 1994 {published data only}
-
- Working Group for the Study of Transdermal Nicotine. Nicotine replacement therapy for patients with coronary artery disease. Working Group for the Study of Transdermal Nicotine in Patients with Coronary artery disease. Archives of Internal Medicine 1994;154:989‐95. - PubMed
References to ongoing studies
NCT01010477 {unpublished data only}
-
- NCT01010477. Trial of nicotine nasal spray as an aid for smoking cessation in schizophrenia. ClinicalTrials.gov/show/NCT01010477 2009.
NCT01484340 {published data only}
-
- NCT01484340. A smoking cessation trial in HIV‐infected patients in South Africa. ClinicalTrials.gov/show/NCT01484340 (first received 2 December 2011).
NCT02918500 {published data only}
-
- NCT02918500. Effect of pre‐op NRT on peri‐operative complications and long‐term abstinence: a pilot trial in patients undergoing CABG surgery. ClinicalTrials.gov/show/NCT02918500 (first received 29 September 2016).
Additional references
Alpert 2012
-
- Alpert HR, Connolly GN, Biener L. A prospective cohort study challenging the effectiveness of population‐based medical intervention for smoking cessation. Tobacco Control 2012; Vol. 22, issue 1:32‐7. - PubMed
Altman 2004
Bailey 2012
Cahill 2015
Cahill 2016
Carson 2012
Cepeda‐Benito 2004
-
- Cepeda‐Benito A, Reynoso JT, Erath S. Meta‐analysis of the efficacy of nicotine replacement therapy for smoking cessation: differences between men and women. Journal of Consulting and Clinical Psychology 2004;72:712‐22. - PubMed
Clavel 1984
-
- Clavel F, Benhamou S. Tobacco withdrawal. Comparison of the efficacy of various methods. Intermediate results of a comparative study [Désintoxcation tabagique. Comparison de l'efficacité de differentes méthodes. Résultats intermédiaires d'une étude comparative]. Presse Médicale 1984;13:975‐7. - PubMed
Coleman 2015
CONSORT 2001
-
- Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Annals of Internal Medicine 2001;134:663‐94. - PubMed
Cooper 2003
-
- Cooper TV, Montgomery GV, Debon MW, Zbikowski SM, Klesges RC, Johnson KC. The effects of PPA and nicotine gum on cessation rates and post cessation weight gain in women [POS3‐46]. Society for Research on Nicotine and Tobacco 9th Annual Meeting; 2003; New Orleans, LA, USA. 2003.
Daughton 1999
-
- Daughton DM, Fortmann SP, Glover ED, Hatsukami DK, Heatley SA, Lichtenstein E, et al. The smoking cessation efficacy of varying doses of nicotine patch delivery systems 4 to 5 years post‐quit day. Preventive Medicine 1999;28:113‐8. - PubMed
Deeks 2005
-
- Deeks JJ, Higgins JPT, Altman DG, editor(s), on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. in: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Available from handbook.cochrane.org.
Durcan 2002
-
- Durcan MJ, White J, Jorenby DE, Fiore MC, Rennard SI, Leischow SJ, et al. Impact of prior nicotine replacement therapy on smoking cessation efficacy. American Journal of Health Behaviors 2002;26:213‐20. - PubMed
Etter 2006
Etter 2007
-
- Etter JF, Burri M, Stapleton J. The impact of pharmaceutical company funding on results of randomized trials of nicotine replacement therapy for smoking cessation: a meta‐analysis (PA9‐6). Addiction 2007;102:815‐22. - PubMed
Fagerström 2003
-
- Fagerström KO. Clinical treatment of tobacco dependence: The endurance of pharmacologic efficacy. Journal of Clinical Psychiatry Monograph 2003;18:35‐40.
Fanshawe 2017
Fiore 1992
-
- Fiore MC, Jorenby DE, Baker TB, Kenford SL. Tobacco dependence and the nicotine patch. Clinical guidelines for effective use. JAMA 1992;268:2687‐94. - PubMed
Fiore 2008
-
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service, May 2008.
Greenland 1998
-
- Greenland S, Satterfield MH, Lanes SF. A meta‐analysis to assess the incidence of adverse effects associated with the transdermal nicotine patch. Drug Safety 1998;18:297‐308. - PubMed
Hartmann‐Boyce 2016
Henningfield 2005
-
- Henningfield JE, Fant RV, Buchhalter AR, Stitzer ML. Pharmacotherapy for nicotine dependence. CA Cancer Journal for Clinicians 2005;55:281‐99. - PubMed
Higgins 2003
Hughes 2001
-
- Hughes JR. The effectiveness of over‐the‐counter nicotine replacement: a rebuttal. Drug and Alcohol Review 2001;20:319‐22.
Hughes 2004
-
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long‐term abstinence among untreated smokers. Addiction 2004;99:29‐38. - PubMed
Hughes 2014
Italy ISS 2004
-
- Osservatorio Fumo, Alcol e Droga. Linee Guida Cliniche per Promuovere la Cessazione dell’Abitudine al Fumo. Rome: Istituto Superiore di Sanita, 2004.
Jensen 1990
Joseph 1999
-
- Joseph AM, Antonnucio DO. Lack of efficacy of transdermal nicotine in smoking cessation. New England Journal of Medicine 1999;341:1157‐8. - PubMed
Joseph 2003
-
- Joseph AM, Fu SS. Safety issues in pharmacotherapy for smoking in patients with cardiovascular disease. Progress in Cardiovascular Diseases 2003;45:429‐41. - PubMed
Kasza 2012
Kotz 2014
Le Foll 2005
-
- Foll B, Melihan‐Cheinin P, Rostoker G, Lagrue G. Smoking cessation guidelines: evidence‐based recommendations of the French Health Products Safety Agency. European Psychiatry 2005;20:431‐41. - PubMed
Lindson‐Hawley 2016
Meine 2005
-
- Meine TJ, Patel MR, Washam JB, Pappas PA, Jollis JG. Safety and effectiveness of transdermal nicotine patch in smokers admitted with acute coronary syndromes. American Journal of Cardiology 2005;95:976‐8. - PubMed
Mikkelsen 1994
-
- Mikkelsen KL, Tønnesen P, Norregaard J. Three‐year outcome of two‐ and three‐year sustained abstainers from a smoking cessation study with nicotine patches. Journal of Smoking‐Related Disorders 1994;5:95‐100.
Mills 2010
Mills 2014
Mooney 2004
-
- Mooney M, White T, Hatsukami D. The blind spot in the nicotine replacement therapy literature: assessment of the double‐blind in clinical trials. Addictive Behaviors 2004;29(4):673‐84. - PubMed
Muller 1990
-
- Muller P, Abelin T, Ehrsam R, Imhof P, Howald H, Mauli D. The use of transdermal nicotine in smoking cessation. Lung 1990;168:445‐53. - PubMed
Munafó 2004a
-
- Munafó M, Bradburn M, Bowes L, David S. Are there sex differences in transdermal nicotine replacement therapy patch efficacy? A meta‐analysis. Nicotine & Tobacco Research 2004;6:769‐76. - PubMed
Munafó 2004b
-
- Munafó M, Bradburn M, Bowes L, David S. Investigating subgroups in smoking cessation treatment response: Response to Perkins. Nicotine & Tobacco Research 2004;6:865‐7.
NICE 2008
-
- National Institute for Health and Clinical Excellence. Public Health Guideline [PH10] Stop Smoking Services. National Institute for Health and Clinical Excellence, 2008.
NZ MoH 2014
-
- Ministry of Health. The New Zealand Guidelines for Helping People to Stop Smoking.. Wellington, New Zealand: Ministry of Health, 2014.
Ockene 1994
-
- Ockene JK, Kristeller J, Pbert L, Hebert JR, Luippold R, Goldberg RJ, et al. The physician‐delivered smoking intervention project: can short‐term interventions produce long‐term effects for a general outpatient population?. Health Psychology 1994;13:278‐81. - PubMed
Palmer 1992
-
- Palmer KJ, Buckley MM, Faulds D. Transdermal nicotine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy as an aid to smoking cessation. Drugs 1992;44:498‐529. - PubMed
Perkins 2004
-
- Perkins KA. Obstacles to determining individual differences in the efficacy of smoking cessation medications. Nicotine & Tobacco Research 2004;6:765‐7. - PubMed
Pierce 2002
-
- Pierce JP, Gilpin EA. Impact of over‐the‐counter sales on effectiveness of pharmaceutical aids for smoking cessation. JAMA 2002;288:1260‐4. - PubMed
Richmond 1997
-
- Richmond RL, Kehoe L, Neto ACdA. Effectiveness of a 24‐hour transdermal nicotine patch in conjunction with a cognitive behavioural programme: One year outcome. Addiction 1997;92:27‐31. - PubMed
Schneider 1983
-
- Schneider NG, Jarvik ME, Forsythe AB, Read LL, Elliott ML, Schweiger A. Nicotine gum in smoking cessation: a placebo‐controlled, double‐blind trial. Addictive Behaviors 1983;8:253‐61. - PubMed
Stapleton 1998
Stapleton 2012
-
- Stapleton, JA. Perverse conclusion from results. Tobacco Control 2012:np.
Suissa 2017
-
- Suissa K, Larivière J, Eisenberg MJ, Eberg M, Gore GC, Grad R, et al. Efficacy and safety of smoking cessation interventions in patients with cardiovascular disease: a network meta‐analysis of randomized controlled trials. Circulation: Cardiovascular Quality and Outcomes 2017;10(1):e002458. - PubMed
TNWG 1994
-
- Transdermal Nicotine Working Group. Nicotine replacement therapy for patients with coronary artery disease. Working Group for the Study of Transdermal Nicotine in Patients with Coronary Artery Disease. Archives of Internal Medicine 1994;154:989‐95. - PubMed
Tønnesen 1992
-
- Tønnesen P, Norregaard J, Sawe U. Two‐year outcome in a smoking cessation trial with a nicotine patch. Journal of Smoking‐Related Disorders 1992;3:241‐5.
Wallstrom 1999
-
- Wallstrom M, Sand L, Nilsson F, Hirsch JM. The long‐term effect of nicotine on the oral mucosa. Addiction 1999;94:417‐23. - PubMed
Walsh 2000
-
- Walsh RA, Penman AG. The effectiveness of nicotine replacement therapy over‐the‐counter. Drug and Alcohol Review 2000;19:243‐7.
Walsh 2001
-
- Walsh RA, Penman AG. The effectiveness of over‐the‐counter nicotine replacement: reply to Hughes. Drug and Alcohol Review 2001;20:322‐4.
Walsh 2007
West 2001
-
- West R, .Shiffman S. Effect of oral nicotine dosing forms on cigarette withdrawal symptoms and craving: a systematic review. Psychopharmacology Berl 2001;155:115‐22. - PubMed
West 2007
Woolacott 2002
-
- Woolacott NF, Jones L, Forbes CA, Mather LC, Sowden AJ, Song FJ, et al. The clinical effectiveness and cost‐effectiveness of bupropion and nicotine replacement therapy for smoking cessation: a systematic review and economic evaluation. Health Technology Assessment 2002;6:1‐245. - PubMed
Zwar 2011
-
- Zwar N, Borland R, Peters M, Litt J, Bell J, Caldwell B, et al. Supporting Smoking Cessation: A Guide for Health Professionals. Melbourne: The Royal Australian College of General Practitioners, 2011.
References to other published versions of this review
Silagy 1994a
-
- Silagy C, Mant D, Fowler G, Lodge M. Meta‐analysis on efficacy of nicotine replacement therapies in smoking cessation. Lancet 1994;343:139‐42. - PubMed
Silagy 1994b
-
- Silagy C, Mant D, Fowler G, Lancaster T. The effectiveness of nicotine replacement therapies in smoking cessation. Online Journal of Current Clinical Trials 1994;Doc No:113. - PubMed
Silagy 1996
Silagy 2001
Silagy 2002
Silagy 2004
Stead 2008
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

