MicroRNAs as potential liquid biopsy biomarkers in colorectal cancer: A systematic review
- PMID: 29852194
- PMCID: PMC7286572
- DOI: 10.1016/j.bbcan.2018.05.006
MicroRNAs as potential liquid biopsy biomarkers in colorectal cancer: A systematic review
Abstract
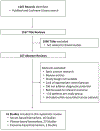
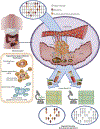
Emerging evidence has demonstrated the feasibility of circulating miRNAs as robust non-invasive biomarkers for the diagnosis in colorectal cancer. The use of circulating miRNAs for the early detection of colorectal cancer (CRC) is of particular interest as it can offer a potential complementary approach to screening colonoscopy. However, the development of circulating miRNAs as "liquid biopsy" biomarkers for development into clinical screening tests has been hampered by several issues. In this article, we summarize the status of this field for the clinical utilization of miRNA biomarkers as liquid biopsies in colorectal cancer (CRC) and discuss their applications as screening tests for patients with colorectal adenoma (CRA) and CRC. Herein, we undertook a systematic search for citations in PubMed and the Cochrane Database from January 1, 2002 through December 31, 2017 as electronic sources for this study. All published studies were screened with no restriction on language, date, or country. We used database-specific combinations of the following index terms and text words, including: microRNA, colorectal cancer, serum, plasma, and exosomes. Based upon these searches, we summarize the progress and salient features of the current state of knowledge of miRNA diagnostic biomarkers in CRC, and focuses on the articles that attempt to optimize ideal methodologies to further advance their as liquid biopsies for clinical use. We conclude that the field of noncoding RNAs, particularly for the clinical use of miRNAs as liquid biopsy assays is maturing rapidly, and it is highly promising that these genomic signatures will likely be developed into clinically-viable tests for the early detection and clinical management of patients with colorectal cancer in the not so distant future.
Keywords: Biomarker; Colorectal cancer; Diagnosis; MicroRNA; Screening.
Copyright © 2018 Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures
Similar articles
-
Global and targeted circulating microRNA profiling of colorectal adenoma and colorectal cancer.Cancer. 2018 Feb 15;124(4):785-796. doi: 10.1002/cncr.31062. Epub 2017 Nov 7. Cancer. 2018. PMID: 29112225 Free PMC article.
-
Plasma MicroRNA Signature Validation for Early Detection of Colorectal Cancer.Clin Transl Gastroenterol. 2019 Jan;10(1):e00003. doi: 10.14309/ctg.0000000000000003. Clin Transl Gastroenterol. 2019. PMID: 30702491 Free PMC article.
-
Combined Plasma MicroRNA and Fecal Occult Blood Tests in Early Detection of Colorectal Cancer.Clin Lab. 2019 May 1;65(5). doi: 10.7754/Clin.Lab.2018.180926. Clin Lab. 2019. PMID: 31115212
-
Circulating biomarkers for early detection and clinical management of colorectal cancer.Mol Aspects Med. 2019 Oct;69:107-122. doi: 10.1016/j.mam.2019.06.002. Epub 2019 Jun 14. Mol Aspects Med. 2019. PMID: 31189073 Review.
-
Non-coding RNAs as Biomarkers for Colorectal Cancer Screening and Early Detection.Adv Exp Med Biol. 2016;937:153-70. doi: 10.1007/978-3-319-42059-2_8. Adv Exp Med Biol. 2016. PMID: 27573899 Review.
Cited by
-
Non-Invasive Colorectal Cancer Screening: An Overview.Gastrointest Tumors. 2020 Jul;7(3):62-73. doi: 10.1159/000507701. Epub 2020 May 20. Gastrointest Tumors. 2020. PMID: 32903904 Free PMC article. Review.
-
M2 macrophage-derived exosomal microRNA-155-5p promotes the immune escape of colon cancer by downregulating ZC3H12B.Mol Ther Oncolytics. 2021 Feb 6;20:484-498. doi: 10.1016/j.omto.2021.02.005. eCollection 2021 Mar 26. Mol Ther Oncolytics. 2021. PMID: 33718596 Free PMC article.
-
Biological surface properties in extracellular vesicles and their effect on cargo proteins.Sci Rep. 2019 Sep 10;9(1):13048. doi: 10.1038/s41598-019-47598-3. Sci Rep. 2019. PMID: 31506490 Free PMC article.
-
Perspectives of the Application of Liquid Biopsy in Colorectal Cancer.Biomed Res Int. 2020 Mar 9;2020:6843180. doi: 10.1155/2020/6843180. eCollection 2020. Biomed Res Int. 2020. PMID: 32258135 Free PMC article. Review.
-
Comprehensive analysis of 5-hydroxymethylcytosine in zw10 kinetochore protein as a promising biomarker for screening and diagnosis of early colorectal cancer.Clin Transl Med. 2020 Jul;10(3):e125. doi: 10.1002/ctm2.125. Epub 2020 Jul 6. Clin Transl Med. 2020. PMID: 32628818 Free PMC article.
References
-
- Siegel RL, Miller KD, Jemal A, Cancer statistics, 2016, CA: a cancer journal for clinicians, 66 (2016) 7–30. - PubMed
-
- Walsh JM, Terdiman JP, Colorectal cancer screening: clinical applications, JAMA : the journal of the American Medical Association, 289 (2003) 1297–1302. - PubMed
-
- Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, Rassenti L, Kipps T, Negrini M, Bullrich F, Croce CM, Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia, Proceedings of the National Academy of Sciences of the United States of America, 99 (2002) 15524–15529. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

