Tocilizumab discontinuation after attaining remission in patients with rheumatoid arthritis who were treated with tocilizumab alone or in combination with methotrexate: results from a prospective randomised controlled study (the second year of the SURPRISE study)
- PMID: 29853455
- PMCID: PMC6104678
- DOI: 10.1136/annrheumdis-2018-213416
Tocilizumab discontinuation after attaining remission in patients with rheumatoid arthritis who were treated with tocilizumab alone or in combination with methotrexate: results from a prospective randomised controlled study (the second year of the SURPRISE study)
Abstract
Objective: To evaluate the sustained remission and low disease activity after discontinuation of tocilizumab in patients with rheumatoid arthritis who were treated with tocilizumab alone or in combination with methotrexate.
Methods: The SURPRISE study was a 2-year, open-label randomised controlled study. Among patients who had been randomised to additional tocilizumab (ADD-ON) or switch to tocilizumab (SWITCH) in the first year, those who achieved remission based on the disease activity score for 28 joints (DAS28-ESR<2.6) discontinued tocilizumab at week 52 and were observed for the following 52 weeks. The endpoint of the second year included tocilizumab-free remission and low disease-activity rates, functional outcome, radiological outcomes assessed with the modified total Sharp score (mTSS) and safety. The efficacy of reinstituted tocilizumab/methotrexate was also evaluated.
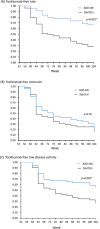
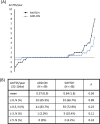
Results: A total of 105 patients who achieved remission at week 52 discontinued tocilizumab; 51 in ADD-ON continued methotrexate and 54 in SWITCH received no disease-modifying antirheumatic drugs. Sustained DAS28 low disease-activity rates were significantly higher in ADD-ON than in SWITCH (55%vs27%, p=0.005). Sustained remission rates at week 104 were 24% for ADD-ON and 14% for SWITCH (p=0.29). Radiological progression was comparable between both groups (mTSS; 0.37vs0.64, p=0.36). The restart of tocilizumab induced remission in all except two patients after 36 weeks, irrespective of concomitant methotrexate.
Conclusion: Sustained low disease activity after tocilizumab discontinuation could be maintained with continued methotrexate in more than half of the patients. Retreatment with tocilizumab led to remission in more than 90% of patients.
Trial registration number: NCT01120366; Results.
Keywords: dmards (biologic); methotrexate; rheumatoid arthritis.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: YK has received grants or speaking fees from from AbbVie, Astellas, Ayumi, Bristol-Myers Squibb, Chugai, Eisai, Eli Lilly, Hisamitsu, Jansen, Kissei, Pfizer, Sanofi, Takeda, Tanabe-Mitsubishi and UCB. MK has received research grants or lecture fees from GlaxoSmithKline K.K. and Actelion Pharmaceuticals. YT has received grants or speaking fees from Mitsubishi-Tanabe, Takeda, Bristol-Myers, Chugai, Astellas, AbbVie, MSD, Eli Lilly, YL Biologics, Daiichi-Sankyo, Sanofi, Janssen, Pfizer, Kyowa-Kirin, Eisai and Ono. HK-H has received speaking fees from Chugai, Astellas and Bristol-Myers Squibb. KA has received research grants from Chugai Pharmaceutical Co. and speaking fees from Daiichi-Sankyo, Eli Lilly, Pfizer Japan and Tanabe-Mitsubishi. MM is an employee of the Japanese Red Cross Society. YM has received grants, instructor fees or speaking fees from Chugai, Ono, Daiichi-Sankyo, Teijin, Eisai, Nippon Kayaku, Tanabe-Mitsubishi, Kissei, Janssen, Eisai, Astellas, Ayumi, Takeda, UCB, Sanofi and Eli- Lilly. HY has received grants or speaking fees from Bristol-Myers Squibb, Takeda, Japan Blood Products Organization, Eizai, Daiichi-Sankyo, Novartis, Chugai and Actelion. SH has received grants, lecture fees or speaking fees from AbbVie, Astellas, Ayumi, Bristol-Myers Squibb, Chugai, Eisai, Eli Lilly, Jansen, Kissei, Pfizer, Sanofi, Takeda, Tanabe-Mitsubishi and UCB. ET received speaking fees from AbbVie, Ayumi Pharmaceutical, Bristol-Myers Squibb, Chugai Pharmaceutical, Eisai Pharmaceutical, Nippon Kayaku, Pfizer, Takeda Pharmaceutical and UCB Pharma. HY has received research grants, consultant fees or speaking fees from MSD, Astellas, AbbVie, Bristol-Myers Squibb, Kaken, UCB, Ono, Ayumi, Eisai, Daiichi-Sankyo, Takeda, Tanabe-Mitsubishi, Chugai, Teijin, Torii, Nipponshinyaku, Pfizer, YL Biologics and Nipponkayaku. KY has received grants or speaking fees from Astellas Pharmaceutical, Chugai Pharmaceutical, Eizai Pharmaceutical, Immunofuture Inc, Mitsubishi Tanabe Pharma Corporation, Santen Pharmaceutical, Pfizer Japan Inc, AbbVie GK, Bristol–Myers KK, Diaichi Sankyo, Eli Lilly, Sanofi, Janssen and UCB. TT has received research grants or speaking fees from Astellas Pharma Inc, Bristol–Myers K.K., Chugai Pharmaceutical Co, Ltd, Daiichi Sankyo Co, Ltd, Takeda Pharmaceutical Co, Ltd, Teijin Pharma Ltd, AbbVie GK, Asahikasei Pharma Corp, Mitsubishi Tanabe Pharma, Astra Zeneca KK, Eli Lilly Japan KK, Novartis Pharma KK, AbbVie GK, Nipponkayaku Co, Ltd, Janssen, Pharmaceutical KK, Taiho Pharmaceutical Co, Ltd and Pfizer Japan Inc. MI and NM have nothing to declare.
Figures


References
-
- Smolen JS, Landewé R, Johannes Bijlsma J, et al. . EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

