DIACYLGLYCEROL ACYLTRANSFERASE and DIACYLGLYCEROL KINASE Modulate Triacylglycerol and Phosphatidic Acid Production in the Plant Response to Freezing Stress
- PMID: 29853600
- PMCID: PMC6053003
- DOI: 10.1104/pp.18.00402
DIACYLGLYCEROL ACYLTRANSFERASE and DIACYLGLYCEROL KINASE Modulate Triacylglycerol and Phosphatidic Acid Production in the Plant Response to Freezing Stress
Erratum in
-
CORRECTION: Vol. 177: 1303-1318, 2018.Plant Physiol. 2018 Nov;178(3):1424-1425. doi: 10.1104/pp.18.01200. Plant Physiol. 2018. PMID: 30425160 Free PMC article. No abstract available.
Abstract
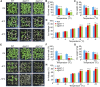
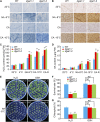
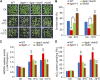
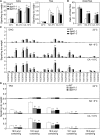
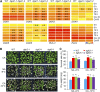
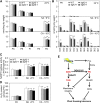
Plants accumulate the lipids phosphatidic acid (PA), diacylglycerol (DAG), and triacylglycerol (TAG) during cold stress, but how plants balance the levels of these lipids to mediate cold responses remains unknown. The enzymes ACYL-COENZYME A:DIACYLGLYCEROL ACYLTRANSFERASE (DGAT) and DIACYLGLYCEROL KINASE (DGK) catalyze the conversion of DAG to TAG and PA, respectively. Here, we show that DGAT1, DGK2, DGK3, and DGK5 contribute to the response to cold in Arabidopsis (Arabidopsis thaliana). With or without cold acclimation, the dgat1 mutants exhibited higher sensitivity upon freezing exposure compared with the wild type. Under cold conditions, the dgat1 mutants showed reduced expression of C-REPEAT/DRE BINDING FACTOR2 and its regulons, which are essential for the acquisition of cold tolerance. Lipid profiling revealed that freezing significantly increased the levels of PA and DAG while decreasing TAG in the rosettes of dgat1 mutant plants. During freezing stress, the accumulation of PA in dgat1 plants stimulated NADPH oxidase activity and enhanced RbohD-dependent hydrogen peroxide production compared with the wild type. Moreover, the cold-inducible transcripts of DGK2, DGK3, and DGK5 were significantly more up-regulated in the dgat1 mutants than in the wild type during cold stress. Consistent with this observation, dgk2, dgk3, and dgk5 knockout mutants showed improved tolerance and attenuated PA production in response to freezing temperatures. Our findings demonstrate that the conversion of DAG to TAG by DGAT1 is critical for plant freezing tolerance, acting by balancing TAG and PA production in Arabidopsis.
© 2018 American Society of Plant Biologists. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

