Increased Microglial Activity, Impaired Adult Hippocampal Neurogenesis, and Depressive-like Behavior in Microglial VPS35-Depleted Mice
- PMID: 29853629
- PMCID: PMC6021995
- DOI: 10.1523/JNEUROSCI.3621-17.2018
Increased Microglial Activity, Impaired Adult Hippocampal Neurogenesis, and Depressive-like Behavior in Microglial VPS35-Depleted Mice
Abstract
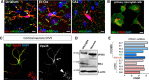
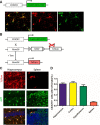
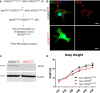
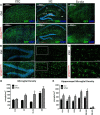
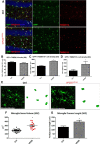
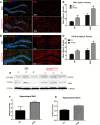
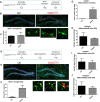
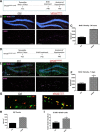
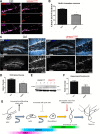
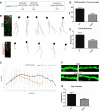
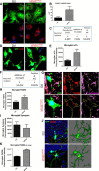
Vacuolar sorting protein 35 (VPS35) is a critical component of retromer, which is essential for selective endosome-to-Golgi retrieval of membrane proteins. VPS35 deficiency is implicated in neurodegenerative disease pathology, including Alzheimer's disease (AD). However, exactly how VPS35 loss promotes AD pathogenesis remains largely unclear. VPS35 is expressed in various types of cells in the brain, including neurons and microglia. Whereas neuronal VPS35 plays a critical role in preventing neurodegeneration, the role of microglial VPS35 is largely unknown. Here we provide evidence for microglial VPS35's function in preventing microglial activation and promoting adult hippocampal neurogenesis. VPS35 is expressed in microglia in various regions of the mouse brain, with a unique distribution pattern in a brain region-dependent manner. Conditional knocking out of VPS35 in microglia of male mice results in regionally increased microglial density and activity in the subgranular zone of the hippocampal dentate gyrus (DG), accompanied by elevated neural progenitor proliferation, but decreased neuronal differentiation. Additionally, newborn neurons in the mutant DG show impaired dendritic morphology and reduced dendritic spine density. When examining the behavioral phenotypes of these animals, microglial VPS3S-depleted mice display depression-like behavior and impairment in long-term recognition memory. At the cellular level, VPS35-depleted microglia have grossly enlarged vacuolar structures with increased phagocytic activity toward postsynaptic marker PSD95, which may underlie the loss of dendritic spines observed in the mutant DG. Together, these findings identify an important role of microglial VPS35 in suppressing microglial activation and promoting hippocampal neurogenesis, which are both processes involved in AD pathogenesis.SIGNIFICANCE STATEMENT The findings presented here provide the first in vivo evidence that Vacuolar sorting protein 35 (VPS35)/retromer is essential for regulating microglial function and that when microglial retromer mechanics are disrupted, the surrounding brain tissue can be affected in a neurodegenerative manner. These findings present a novel, microglial-specific role of VPS35 and raise multiple questions regarding the mechanisms underlying our observations. These findings also have myriad implications for the field of retromer research and the role of retromer dysfunction in neurodegenerative pathophysiology. Furthermore, they implicate a pivotal role of microglia in the regulation of adult hippocampal neurogenesis and the survival/integration of newborn neurons in the adult hippocampus.
Keywords: Alzheimer's disease; VPS35; hippocampus; microglia; neurogenesis; retromer.
Copyright © 2018 the authors 0270-6474/18/385949-20$15.00/0.
Figures

References
-
- Bennett ML, Bennett FC, Liddelow SA, Ajami B, Zamanian JL, Fernhoff NB, Mulinyawe SB, Bohlen CJ, Adil A, Tucker A, Weissman IL, Chang EF, Li G, Grant GA, Hayden Gephart MG, Barres BA (2016) New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci U S A 113:E1738–E1746. 10.1073/pnas.1525528113 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
