Organoid Profiling Identifies Common Responders to Chemotherapy in Pancreatic Cancer
- PMID: 29853643
- PMCID: PMC6125219
- DOI: 10.1158/2159-8290.CD-18-0349
Organoid Profiling Identifies Common Responders to Chemotherapy in Pancreatic Cancer
Abstract
Pancreatic cancer is the most lethal common solid malignancy. Systemic therapies are often ineffective, and predictive biomarkers to guide treatment are urgently needed. We generated a pancreatic cancer patient-derived organoid (PDO) library that recapitulates the mutational spectrum and transcriptional subtypes of primary pancreatic cancer. New driver oncogenes were nominated and transcriptomic analyses revealed unique clusters. PDOs exhibited heterogeneous responses to standard-of-care chemotherapeutics and investigational agents. In a case study manner, we found that PDO therapeutic profiles paralleled patient outcomes and that PDOs enabled longitudinal assessment of chemosensitivity and evaluation of synchronous metastases. We derived organoid-based gene expression signatures of chemosensitivity that predicted improved responses for many patients to chemotherapy in both the adjuvant and advanced disease settings. Finally, we nominated alternative treatment strategies for chemorefractory PDOs using targeted agent therapeutic profiling. We propose that combined molecular and therapeutic profiling of PDOs may predict clinical response and enable prospective therapeutic selection.Significance: New approaches to prioritize treatment strategies are urgently needed to improve survival and quality of life for patients with pancreatic cancer. Combined genomic, transcriptomic, and therapeutic profiling of PDOs can identify molecular and functional subtypes of pancreatic cancer, predict therapeutic responses, and facilitate precision medicine for patients with pancreatic cancer. Cancer Discov; 8(9); 1112-29. ©2018 AACR.See related commentary by Collisson, p. 1062This article is highlighted in the In This Issue feature, p. 1047.
©2018 American Association for Cancer Research.
Conflict of interest statement
Figures



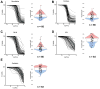

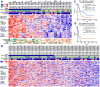
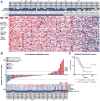
Comment in
-
Organoidomics - falling star or new galaxy in pancreatic cancer?Nat Rev Gastroenterol Hepatol. 2018 Oct;15(10):586-587. doi: 10.1038/s41575-018-0052-3. Nat Rev Gastroenterol Hepatol. 2018. PMID: 30046146 No abstract available.
-
Bringing Pancreas Cancer into the Lab.Cancer Discov. 2018 Sep;8(9):1062-1063. doi: 10.1158/2159-8290.CD-18-0811. Cancer Discov. 2018. PMID: 30181169
References
-
- Khorana AA, Mangu PB, Berlin J, Engebretson A, Hong TS, Maitra A, et al. Potentially Curable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34:2541–56. - PubMed
-
- Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg. 2006;10:1199–210. discussion 210-1. - PubMed
-
- Groot VP, Rezaee N, Wu W, Cameron JL, Fishman EK, Hruban RH, et al. Patterns, Timing, and Predictors of Recurrence Following Pancreatectomy for Pancreatic Ductal Adenocarcinoma. Ann Surg. 2017 - PubMed
-
- Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011–24. - PubMed
-
- Sinn M, Bahra M, Liersch T, Gellert K, Messmann H, Bechstein W, et al. CONKO-005: Adjuvant Chemotherapy With Gemcitabine Plus Erlotinib Versus Gemcitabine Alone in Patients After R0 Resection of Pancreatic Cancer: A Multicenter Randomized Phase III Trial. J Clin Oncol. 2017;35:3330–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P20 CA192996/CA/NCI NIH HHS/United States
- R01 CA188134/CA/NCI NIH HHS/United States
- R01 CA202762/CA/NCI NIH HHS/United States
- R01 CA212600/CA/NCI NIH HHS/United States
- R01 CA190092/CA/NCI NIH HHS/United States
- K99 CA204725/CA/NCI NIH HHS/United States
- P20 CA192994/CA/NCI NIH HHS/United States
- K08 CA218420/CA/NCI NIH HHS/United States
- U01 CA224013/CA/NCI NIH HHS/United States
- P50 CA101955/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- U10 CA180944/CA/NCI NIH HHS/United States
- P30 CA045508/CA/NCI NIH HHS/United States
- UL1 TR001102/TR/NCATS NIH HHS/United States
- P01 CA013106/CA/NCI NIH HHS/United States
- T32 CA148056/CA/NCI NIH HHS/United States
- R01 CA101973/CA/NCI NIH HHS/United States
- P50 CA127297/CA/NCI NIH HHS/United States
- R50 CA211462/CA/NCI NIH HHS/United States
- U01 CA168409/CA/NCI NIH HHS/United States
- U01 CA210240/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

