Overview and Critical Appraisal of Arterial Spin Labelling Technique in Brain Perfusion Imaging
- PMID: 29853806
- PMCID: PMC5964483
- DOI: 10.1155/2018/5360375
Overview and Critical Appraisal of Arterial Spin Labelling Technique in Brain Perfusion Imaging
Abstract
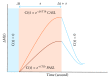
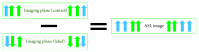
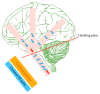
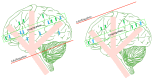
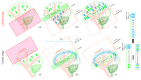
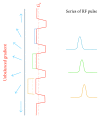
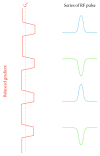
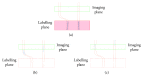
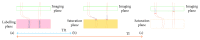
Arterial spin labelling (ASL) allows absolute quantification of CBF via a diffusible intrinsic tracer (magnetically labelled blood water) that disperses from the vascular system into neighbouring tissue. Thus, it can provide absolute CBF quantification, which eliminates the need for the contrast agent, and can be performed repeatedly. This review will focus on the common ASL acquisition techniques (continuous, pulsed, and pseudocontinuous ASL) and how ASL image quality might be affected by intrinsic factors that may bias the CBF measurements. We also provide suggestions to mitigate these risks, model appropriately the acquired signal, increase the image quality, and hence estimate the reliability of the CBF, which consists an important noninvasive biomarker. Emerging methodologies for extraction of new ASL-based biomarkers, such as arterial arrival time (AAT) and arterial blood volume (aBV), will be also briefly discussed.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
