Oscillatory Corticospinal Activity during Static Contraction of Ankle Muscles Is Reduced in Healthy Old versus Young Adults
- PMID: 29853842
- PMCID: PMC5944232
- DOI: 10.1155/2018/3432649
Oscillatory Corticospinal Activity during Static Contraction of Ankle Muscles Is Reduced in Healthy Old versus Young Adults
Abstract
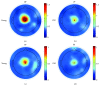
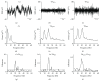
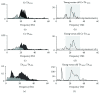
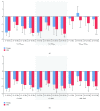
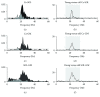
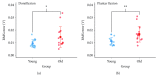
Aging is accompanied by impaired motor function, but age-related changes in neural networks responsible for generating movement are not well understood. We aimed to investigate the functional oscillatory coupling between activity in the sensorimotor cortex and ankle muscles during static contraction. Fifteen young (20-26 yr) and fifteen older (65-73 yr) subjects were instructed to match a target force by performing static ankle dorsi- or plantar flexion, while electroencephalographic (EEG) activity was recorded from the cortex and electromyographic (EMG) activity was recorded from dorsi- (proximal and distal anterior tibia) and plantar (soleus and medial gastrocnemius) flexor muscles. EEG-EMG and EMG-EMG beta band (15-35 Hz) coherence was analyzed as an index of corticospinal activity. Our results demonstrated that beta cortico-, intra-, and intermuscular coherence was reduced in old versus young subjects during static contractions. Old subjects demonstrated significantly greater error than young subjects while matching target forces, but force precision was not related to beta coherence. We interpret this as an age-related decrease in effective oscillatory corticospinal activity during steady-state motor output. Additionally, our data indicate a potential effect of alpha coherence and tremor on performance. These results may be instrumental in developing new interventions to strengthen sensorimotor control in elderly subjects.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

