Guiding Lights in Genome Editing for Inherited Retinal Disorders: Implications for Gene and Cell Therapy
- PMID: 29853845
- PMCID: PMC5964415
- DOI: 10.1155/2018/5056279
Guiding Lights in Genome Editing for Inherited Retinal Disorders: Implications for Gene and Cell Therapy
Abstract
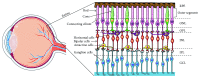
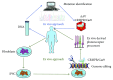
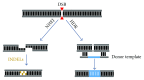
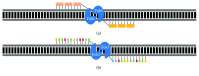
Inherited retinal dystrophies (IRDs) are a leading cause of visual impairment in the developing world. These conditions present an irreversible dysfunction or loss of neural retinal cells, which significantly impacts quality of life. Due to the anatomical accessibility and immunoprivileged status of the eye, ophthalmological research has been at the forefront of innovative and advanced gene- and cell-based therapies, both of which represent great potential as therapeutic treatments for IRD patients. However, due to a genetic and clinical heterogeneity, certain IRDs are not candidates for these approaches. New advances in the field of genome editing using Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) and CRISPR-associated protein (Cas) have provided an accurate and efficient way to edit the human genome and represent an appealing alternative for treating IRDs. We provide a brief update on current gene augmentation therapies for retinal dystrophies. Furthermore, we discuss recent advances in the field of genome editing and stem cell technologies, which together enable precise and personalized therapies for patients. Lastly, we highlight current technological limitations and barriers that need to be overcome before this technology can become a viable treatment option for patients.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

