Simultaneous vs. sequential treatment for smoking and weight management in tobacco quitlines: 6 and 12 month outcomes from a randomized trial
- PMID: 29855294
- PMCID: PMC5984316
- DOI: 10.1186/s12889-018-5574-7
Simultaneous vs. sequential treatment for smoking and weight management in tobacco quitlines: 6 and 12 month outcomes from a randomized trial
Abstract
Background: Smoking cessation often results in weight gain which discourages many smokers from quitting and can increase health risks. Treatments to reduce cessation-related weight gain have been tested in highly controlled trials of in-person treatment, but have never been tested in a real-world setting, which has inhibited dissemination.
Methods: The Best Quit Study (BQS) is a replication and "real world" translation using telephone delivery of a prior in-person efficacy trial.
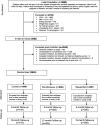
Design: randomized control trial in a quitline setting. Eligible smokers (n = 2540) were randomized to the standard 5-call quitline intervention or quitline plus simultaneous or sequential weight management. Regression analyses tested effectiveness of treatments on self-reported smoking abstinence and weight change at 6 and 12 months.
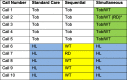
Results: Study enrollees were from 10 commercial employer groups and three state quitlines. Participants were between ages 18-72, 65.8% female, 68.2% white; 23.0% Medicaid-insured, and 76.3% overweight/obese. The follow-up response rate was lower in the simultaneous group than the control group at 6 months (p = 0.01). While a completers analysis of 30-day point prevalence abstinence detected no differences among groups at 6 or 12 months, multiply imputed abstinence showed quit rate differences at 6 months for:simultaneous (40.3%) vs. sequential (48.3%), p = 0.034 and simultaneous vs. control (44.9%), p = 0.043. At 12 months, multiply imputed abstinence, was significantly lower for the simultaneous group (40.7%) vs. control (46.0%), p < 0.05 and vs. sequential (46.3%), p < 0.05. Weight gain at 6 and 12 months was minimal and not different among treatment groups. The sequential group completed fewer total calls (3.75) vs. control (4.16) and vs. simultaneous group (3.83), p = 0.01, and fewer weight calls (0.94) than simultaneous (2.33), p < 0.0001. The number of calls completed predicted 30-day abstinence, p < 0.001, but not weight outcomes.
Discussion: This study offers a model for evaluating population-level public health interventions conducted in partnership with tobacco quitlines.
Conclusions: Simultaneous (vs. sequential) delivery of phone/web weight management with cessation treatment in the quitline setting may adversely affect quit rate. Neither a simultaneous nor sequential approach to addressing weight produced any benefit on suppressing weight gain. This study highlights the need and the challenges of testing intensive interventions in real-world settings.
Trial registration: ClinicalTrials.gov Identifier: NCT01867983 . Registered: May 30, 2013.
Keywords: Quitlines; Smoking; Weight management.
Conflict of interest statement
Authors’ information
BS conducted the original efficacy trial upon which this study was based. BS has written extensively on the topic of post cessation weight gain, published a seminal review of the literature on combined smoking and weight treatments and also on behavioral weight management interventions.
JL was an investigator on the successful and highly influential Diabetes Prevention Program that established a lifestyle intervention was superior to medication in delaying or preventing onset of Type 2diabetes.
Ethics approval and consent to participate
The Western Institutional Review Board and the State of Maryland Institutional Review Board – in accordance with the United States legislation gave consent for the study. Participant consent was obtained verbally via the phone and documented by trained staff. The tobacco cessation and weight management programs are overseen by a clinical team at Alere Wellbeing. All study participants were 18 years of age or older. Alere is a HIPAA covered entity and complies with all HIPAA regulations regarding data security.
Competing interests
The authors at Alere Wellbeing declare that they are employed by Alere Wellbeing (a subsidiary of Optum) which provides tobacco cessation and weight management services to states and commercial clients. They have no other competing interests.
JL declares that she has no competing interests.
HJ declares that he has no competing interests.
BS declares that she has no competing interests.
MT declares that she has no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Finkelstein EA, Fiebelkorn IC, Wang G. National medical spending attributable to overweight and obesity: how much, and who's paying? Health Aff (Millwood). 2003; Suppl Web Exclusives:W3–219-226 - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

