The AWED trial (Applying Wolbachia to Eliminate Dengue) to assess the efficacy of Wolbachia-infected mosquito deployments to reduce dengue incidence in Yogyakarta, Indonesia: study protocol for a cluster randomised controlled trial
- PMID: 29855331
- PMCID: PMC5984439
- DOI: 10.1186/s13063-018-2670-z
The AWED trial (Applying Wolbachia to Eliminate Dengue) to assess the efficacy of Wolbachia-infected mosquito deployments to reduce dengue incidence in Yogyakarta, Indonesia: study protocol for a cluster randomised controlled trial
Abstract
Background: Dengue and other arboviruses transmitted by Aedes aegypti mosquitoes, including Zika and chikungunya, present an increasing public health challenge in tropical regions. Current vector control strategies have failed to curb disease transmission, but continue to be employed despite the absence of robust evidence for their effectiveness or optimal implementation. The World Mosquito Program has developed a novel approach to arbovirus control using Ae. aegypti stably transfected with Wolbachia bacterium, with a significantly reduced ability to transmit dengue, Zika and chikungunya in laboratory experiments. Modelling predicts this will translate to local elimination of dengue in most epidemiological settings. This study protocol describes the first trial to measure the efficacy of Wolbachia in reducing dengue virus transmission in the field.
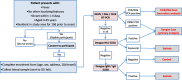
Methods/design: The study is a parallel, two-arm, non-blinded cluster randomised controlled trial conducted in a single site in Yogyakarta, Indonesia. The aim is to determine whether large-scale deployment of Wolbachia-infected Ae. aegypti mosquitoes leads to a measurable reduction in dengue incidence in treated versus untreated areas. The primary endpoint is symptomatic, virologically confirmed dengue virus infection of any severity. The 26 km2 study area was subdivided into 24 contiguous clusters, allocated randomly 1:1 to receive Wolbachia deployments or no intervention. We use a novel epidemiological study design, the cluster-randomised test-negative design trial, in which dengue cases and arbovirus-negative controls are sampled concurrently from among febrile patients presenting to a network of primary care clinics, with case or control status classified retrospectively based on the results of laboratory diagnostic testing. Efficacy is estimated from the odds ratio of Wolbachia exposure distribution (probability of living in a Wolbachia-treated area) among virologically confirmed dengue cases compared to test-negative controls. A secondary per-protocol analysis allows for individual Wolbachia exposure levels to be assessed to account for movements outside the cluster and the heterogeneity in local Wolbachia prevalence among treated clusters.
Discussion: The findings from this study will provide the first experimental evidence for the efficacy of Wolbachia in reducing dengue incidence. Together with observational evidence that is accumulating from pragmatic deployments of Wolbachia in other field sites, this will provide valuable data to estimate the effectiveness of this novel approach to arbovirus control, inform future cost-effectiveness estimates, and guide plans for large-scale deployments in other endemic settings.
Trial registration: ClinicalTrials.gov, identifier: NCT03055585 . Registered on 14 February 2017.
Keywords: Indonesia; Wolbachia; Zika; chikungunya; cluster randomised trial; dengue; test-negative design; vector-borne disease.
Conflict of interest statement
Ethics approval and consent to participate
This trial protocol has been approved by the Universitas Gadjah Mada ethics committee (approval number KE/FK/105/EC/2016) and Monash University Human Research Ethics Committee (approval number 0960). Written informed consent will be obtained from participants, or their guardian where the participant is a minor. In addition, participants aged between 13 and 17 years will be invited to sign an assent form indicating that they understand the research and agree to participate.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

