A common antigenic motif recognized by naturally occurring human VH5-51/VL4-1 anti-tau antibodies with distinct functionalities
- PMID: 29855358
- PMCID: PMC5984341
- DOI: 10.1186/s40478-018-0543-z
A common antigenic motif recognized by naturally occurring human VH5-51/VL4-1 anti-tau antibodies with distinct functionalities
Abstract
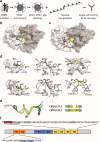
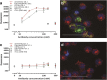
Misfolding and aggregation of tau protein are closely associated with the onset and progression of Alzheimer's Disease (AD). By interrogating IgG+ memory B cells from asymptomatic donors with tau peptides, we have identified two somatically mutated VH5-51/VL4-1 antibodies. One of these, CBTAU-27.1, binds to the aggregation motif in the R3 repeat domain and blocks the aggregation of tau into paired helical filaments (PHFs) by sequestering monomeric tau. The other, CBTAU-28.1, binds to the N-terminal insert region and inhibits the spreading of tau seeds and mediates the uptake of tau aggregates into microglia by binding PHFs. Crystal structures revealed that the combination of VH5-51 and VL4-1 recognizes a common Pro-Xn-Lys motif driven by germline-encoded hotspot interactions while the specificity and thereby functionality of the antibodies are defined by the CDR3 regions. Affinity improvement led to improvement in functionality, identifying their epitopes as new targets for therapy and prevention of AD.
Keywords: Alzheimer’s disease; Antigenic motif; Monoclonal antibody; Tau protein.
Conflict of interest statement
Ethics approval and consent to participate
Brain samples were obtained from The Netherlands Brain Bank (NBB), Netherlands Institute for Neuroscience, Amsterdam. All donors had given written informed consent for brain autopsy and the use of material and clinical information for research purposes. Whole blood from healthy male and female donors was obtained from the San Diego Blood Bank (ages 18–65 years) after informed consent was obtained from the donors.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
-
- Afonine PV, Grosse-Kunstleve RW, Echols N, Headd JJ, Moriarty NW, Mustyakimov M, Terwilliger TC, Urzhumtsev A, Zwart PH, Adams PD. Towards automated crystallographic structure refinement with phenix.Refine. Acta Crystallogr Sect D Biol Crystallogr. 2012;68:352–367. doi: 10.1107/S0907444912001308. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

